当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characteristics and mechanism of low-temperature NO adsorption by activated carbon
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-07-01 , DOI: 10.1016/j.cej.2024.153639 Zhongwei Li , Xingyu Yang , Yutong Wang , Hairui Yang , Qiang Song
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-07-01 , DOI: 10.1016/j.cej.2024.153639 Zhongwei Li , Xingyu Yang , Yutong Wang , Hairui Yang , Qiang Song
|
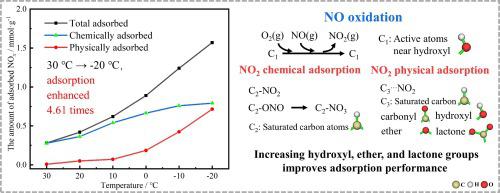
Activated carbon is widely used to purify flue gas in industries like steel and metallurgy, but its ability to adsorb NO at medium to low flue gas temperatures is limited. This study conducted adsorption experiments in different atmospheres within −20 to 30 °C. As the temperature decreased from 30 °C to −20 °C, the NO adsorption amount was increased by 4.61 times. Lowering temperature greatly promoted NO adsorption. Temperature-programmed desorption experiments determined the amount of NO adsorbed through physical adsorption and chemical adsorption. As the temperature decreased from 30 °C to −20 °C, the proportion of physically adsorbed NO to total adsorption amount increased from 2.9 % to 45.5 %, and the proportion of physically adsorbed NO to total physical adsorption amount increased from 0 to71.6 %. Lowering the temperature benefited NO oxidation and NO′s adsorption on activated carbon. Desorption curves of chemically adsorbed NO indicated that a decrease in temperature does not affect the chemical adsorption pathways of NO. Physical adsorption of NO was studied by density functional theory calculations. NO has much stronger physical adsorption than NO and can physically adsorb near saturated carbon atoms, carbonyl groups, ether groups, hydroxyl groups, and lactone groups. At low temperatures, NO is first oxidized to NO on activated carbon and then NO is adsorbed through both physical and chemical pathways. Modifying the surface of activated carbon to increase hydroxyl, ether, and lactone groups can help enhance its performance of low-temperature NO adsorption.
中文翻译:

活性炭低温吸附NO的特性及机理
活性炭广泛应用于钢铁、冶金等行业的烟气净化,但其在中低烟气温度下吸附NO的能力有限。本研究在-20至30°C的不同气氛下进行了吸附实验。随着温度从30℃降低到-20℃,NO吸附量增加了4.61倍。降低温度大大促进了NO的吸附。程序升温脱附实验通过物理吸附和化学吸附测定了NO的吸附量。随着温度从30℃降低到-20℃,物理吸附NO占总吸附量的比例从2.9%增加到45.5%,物理吸附NO占总物理吸附量的比例从0增加到71.6 %。降低温度有利于NO的氧化和NO在活性炭上的吸附。化学吸附NO的解吸曲线表明,温度降低并不影响NO的化学吸附途径。通过密度泛函理论计算研究了NO的物理吸附。 NO的物理吸附性比NO强得多,可以物理吸附近饱和碳原子、羰基、醚基、羟基、内酯基。在低温下,NO首先在活性炭上氧化成NO,然后通过物理和化学途径吸附NO。对活性炭表面进行改性,增加羟基、醚基、内酯基团,有助于增强其低温NO吸附性能。
更新日期:2024-07-01
中文翻译:

活性炭低温吸附NO的特性及机理
活性炭广泛应用于钢铁、冶金等行业的烟气净化,但其在中低烟气温度下吸附NO的能力有限。本研究在-20至30°C的不同气氛下进行了吸附实验。随着温度从30℃降低到-20℃,NO吸附量增加了4.61倍。降低温度大大促进了NO的吸附。程序升温脱附实验通过物理吸附和化学吸附测定了NO的吸附量。随着温度从30℃降低到-20℃,物理吸附NO占总吸附量的比例从2.9%增加到45.5%,物理吸附NO占总物理吸附量的比例从0增加到71.6 %。降低温度有利于NO的氧化和NO在活性炭上的吸附。化学吸附NO的解吸曲线表明,温度降低并不影响NO的化学吸附途径。通过密度泛函理论计算研究了NO的物理吸附。 NO的物理吸附性比NO强得多,可以物理吸附近饱和碳原子、羰基、醚基、羟基、内酯基。在低温下,NO首先在活性炭上氧化成NO,然后通过物理和化学途径吸附NO。对活性炭表面进行改性,增加羟基、醚基、内酯基团,有助于增强其低温NO吸附性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号