当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting chemoselectivity for discrete oligomer synthesis through sequential IrAAC and CuAAC reactions
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-07-02 , DOI: 10.1039/d4py00503a Ningning Song 1 , Lvhao Zhang 1 , Shengtao Ding 1
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-07-02 , DOI: 10.1039/d4py00503a Ningning Song 1 , Lvhao Zhang 1 , Shengtao Ding 1
Affiliation
|
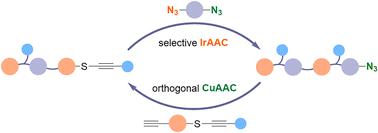
In this study, we introduce the pioneering use of two distinct metal-catalyzed azide–alkyne cycloaddition reactions, CuAAC and IrAAC, for the synthesis of discrete oligomers without requiring protection–deprotection manipulations. The key to this methodology lies in the exceptional chemoselectivity of these reactions towards different alkyne substrates, which ensures their orthogonality. Additionally, the differential reactivity of azido groups in the IrAAC process addresses the common challenge of using excess difunctional monomers within a symmetrical architecture. Remarkably, the discrete oligomer demonstrates facile interpretation in MS/MS analysis due to its two effective fragmentation patterns. This feature not only highlights the potential of this novel synthetic approach for the development of high-density digital polymers but also suggests the broader applicability of triazole-enriched architectures. Further enhancements through side chain modifications with various luminophores and subsequent photophysical studies underscore the potential of this strategy for practical applications.
中文翻译:

通过顺序 IrAAC 和 CuAAC 反应利用化学选择性进行离散低聚物合成
在这项研究中,我们介绍了两种不同的金属催化叠氮-炔环加成反应(CuAAC 和 IrAAC)的开创性用途,用于合成离散低聚物,而无需保护-脱保护操作。该方法的关键在于这些反应对不同炔底物的特殊化学选择性,这确保了它们的正交性。此外,IrAAC 工艺中叠氮基的不同反应性解决了在对称结构中使用过量双官能单体的常见挑战。值得注意的是,由于其两种有效的断裂模式,离散低聚物在 MS/MS 分析中表现出简单的解释。这一特征不仅凸显了这种新颖的合成方法在开发高密度数字聚合物方面的潜力,而且还表明富含三唑的结构具有更广泛的适用性。通过使用各种发光团进行侧链修饰以及随后的光物理研究的进一步增强强调了该策略在实际应用中的潜力。
更新日期:2024-07-03
中文翻译:

通过顺序 IrAAC 和 CuAAC 反应利用化学选择性进行离散低聚物合成
在这项研究中,我们介绍了两种不同的金属催化叠氮-炔环加成反应(CuAAC 和 IrAAC)的开创性用途,用于合成离散低聚物,而无需保护-脱保护操作。该方法的关键在于这些反应对不同炔底物的特殊化学选择性,这确保了它们的正交性。此外,IrAAC 工艺中叠氮基的不同反应性解决了在对称结构中使用过量双官能单体的常见挑战。值得注意的是,由于其两种有效的断裂模式,离散低聚物在 MS/MS 分析中表现出简单的解释。这一特征不仅凸显了这种新颖的合成方法在开发高密度数字聚合物方面的潜力,而且还表明富含三唑的结构具有更广泛的适用性。通过使用各种发光团进行侧链修饰以及随后的光物理研究的进一步增强强调了该策略在实际应用中的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号