当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-light-induced self-catalyzed fluoroalkylation/cyclization of N-arylcinnamamides: synthesis of fluoroalkyl-containing 3,4-disubstituted dihydro-1,5-naphthyridin-2(1H)-ones and 7,8-disubstituted dihydropyrido[3,2-d]pyrimidin-6(5H)-ones
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2024-07-02 , DOI: 10.1039/d4nj01975j Hongmiao Yao 1 , Qianding Zeng 1 , Yiqun Tang 1 , Xiangqiao Yang 1 , Shaodong Wang 1 , Jiangmeng Ren 1 , Bu-Bing Zeng 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2024-07-02 , DOI: 10.1039/d4nj01975j Hongmiao Yao 1 , Qianding Zeng 1 , Yiqun Tang 1 , Xiangqiao Yang 1 , Shaodong Wang 1 , Jiangmeng Ren 1 , Bu-Bing Zeng 1
Affiliation
|
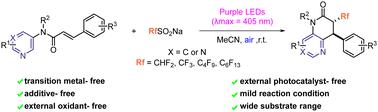
A novel visible-light-mediated fluoroalkylation/cyclization tandem process for constructing fluoroalkyl-containing 3,4-disubstituted dihydro-1,5-naphthyridin-2(1H)-ones and 7,8-disubstituted dihydropyrido[3,2-d]pyrimidin-6(5H)-ones has been explored. This method is compatible with a wide range of N-arylcinnamamides as well as sodium fluoroalkylsulfonates (RfSO2Na, Rf = CHF2, CF3, C4F9, C6F13) and avoids the need for any external oxidant or photocatalyst. Mechanism studies revealed that the singlet oxygen coexists with the superoxide radical anion through energy transfer and single electron transfer processes during the photoredox reaction.
中文翻译:

可见光诱导N-芳基肉桂酰胺的自催化氟烷基化/环化:含氟烷基的3,4-二取代二氢-1,5-萘啶-2(1H)-酮和7,8-二取代二氢吡啶酮的合成[3, 2-d]嘧啶-6(5H)-酮
一种新型可见光介导的氟烷基化/环化串联过程,用于构建含氟烷基的3,4-二取代二氢-1,5-萘啶-2(1H)-酮和7,8-二取代二氢吡啶并[3,2-d]嘧啶-6(5H)-酮已被探索。该方法与多种 N-芳基肉桂酰胺以及氟烷基磺酸钠 (RfSO 2 Na, Rf = CHF 2 , CF 3 , C < b3> F 9 、C 6 F 13 ),并且无需任何外部氧化剂或光催化剂。机理研究表明,在光氧化还原反应过程中,单线态氧通过能量转移和单电子转移过程与超氧自由基阴离子共存。
更新日期:2024-07-02
中文翻译:

可见光诱导N-芳基肉桂酰胺的自催化氟烷基化/环化:含氟烷基的3,4-二取代二氢-1,5-萘啶-2(1H)-酮和7,8-二取代二氢吡啶酮的合成[3, 2-d]嘧啶-6(5H)-酮
一种新型可见光介导的氟烷基化/环化串联过程,用于构建含氟烷基的3,4-二取代二氢-1,5-萘啶-2(1H)-酮和7,8-二取代二氢吡啶并[3,2-d]嘧啶-6(5H)-酮已被探索。该方法与多种 N-芳基肉桂酰胺以及氟烷基磺酸钠 (RfSO 2 Na, Rf = CHF 2 , CF 3 , C < b3> F 9 、C 6 F 13 ),并且无需任何外部氧化剂或光催化剂。机理研究表明,在光氧化还原反应过程中,单线态氧通过能量转移和单电子转移过程与超氧自由基阴离子共存。






























 京公网安备 11010802027423号
京公网安备 11010802027423号