当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quintuple Free‐Radical Therapy: An Ultralong‐Retention FAND for NIR‐Involved Multiple Site‐Acting Hypoxic Tumor Therapy
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-07-01 , DOI: 10.1002/adfm.202401840 Chuang Zhang 1 , Dongxu Zhao 1 , Fang Fang 1 , Lin Zhu 1 , Weiyu Li 1 , Sa Wang 1 , Yueyun Fan 1 , Jiani Yang 1 , Yanhong Liu 2, 3 , Jinfeng Zhang 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-07-01 , DOI: 10.1002/adfm.202401840 Chuang Zhang 1 , Dongxu Zhao 1 , Fang Fang 1 , Lin Zhu 1 , Weiyu Li 1 , Sa Wang 1 , Yueyun Fan 1 , Jiani Yang 1 , Yanhong Liu 2, 3 , Jinfeng Zhang 1
Affiliation
|
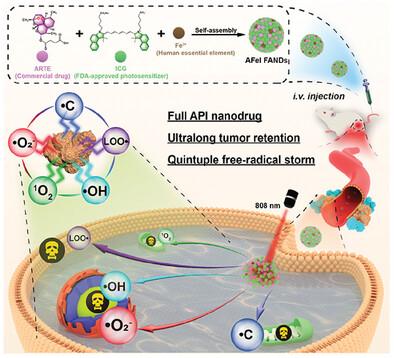
The heavy dependence on intratumoral O2 and H2 O2 availability has greatly restricted ROS‐based therapy. Although O2 /H2 O2 ‐irrelevant free‐radical nanogenerator has garnered tremendous attention as a promising anticancer candidate to overcome the above intrinsic limitations of ROS‐based treatment, the practical therapeutic efficacy of free‐radical therapy is still hindered by limited free‐radical type and inferior tumor retention performance. Herein, inspired by the new concept of full‐API nanodrug (FAND) with 100% active pharmaceutical ingredient (API) content and repurposing of clinical anti‐malaria drug artesunate (ARTE) as an anticancer free‐radical generator, the AFeI FANDs composed of ARTE, human essential Fe3+ , and FDA‐approved fluorescent agent ICG for hypoxic tumor therapy are rationally designed and constructed. Attractively, the completely pharmaceutically active components ARTE, Fe3+ , and ICG can be responsively liberated in the acidic tumor microenvironment and synergistically produce five types of free radicals including •O2 − , •C, •OH, LOO•, and 1 O2 , leading to robust mitochondrial injury, nuclear DNA damage, and lipid peroxides. More importantly, the AFeI FANDs displayed ultralong tumor retention longer than 108 h and favorable tumor suppression outcomes under mild NIR irradiation. Collectively, the presented first paradigm of FAND‐based quintuple free‐radical therapy expands new horizons for the development of clinically transferable nanomedicine for tumor therapy.
中文翻译:

五重自由基疗法:用于近红外多位点作用缺氧肿瘤治疗的超长保留 FAND
对肿瘤内 O2 和 H2O2 可用性的严重依赖极大地限制了基于 ROS 的治疗。尽管与 O2/H2O2 无关的自由基纳米发生器作为一种有前景的抗癌候选药物来克服基于 ROS 的治疗的上述固有局限性而引起了极大的关注,但自由基治疗的实际治疗效果仍然受到有限的自由基类型和限制的阻碍。肿瘤保留性能较差。在此,受具有 100% 活性药物成分 (API) 含量的全 API 纳米药物 (FAND) 新概念的启发,并将临床抗疟疾药物青蒿琥酯 (ARTE) 重新用作抗癌自由基发生器,AFeI FANDs 由ARTE、人体必需的 Fe3+ 和 FDA 批准的用于缺氧肿瘤治疗的荧光剂 ICG 均经过合理设计和构建。有吸引力的是,完全药物活性成分ARTE、Fe3+和ICG可以在酸性肿瘤微环境中响应性释放,并协同产生五种类型的自由基,包括·O2−、·C、·OH、LOO·和1O2,从而产生强大的线粒体损伤、核 DNA 损伤和脂质过氧化物。更重要的是,AFeI FAND 在轻度近红外辐射下表现出超过 108 小时的超长肿瘤保留和良好的肿瘤抑制结果。总的来说,所提出的基于 FAND 的五重自由基疗法的第一个范例为临床可转移的肿瘤治疗纳米药物的开发拓展了新的视野。
更新日期:2024-07-01
中文翻译:

五重自由基疗法:用于近红外多位点作用缺氧肿瘤治疗的超长保留 FAND
对肿瘤内 O2 和 H2O2 可用性的严重依赖极大地限制了基于 ROS 的治疗。尽管与 O2/H2O2 无关的自由基纳米发生器作为一种有前景的抗癌候选药物来克服基于 ROS 的治疗的上述固有局限性而引起了极大的关注,但自由基治疗的实际治疗效果仍然受到有限的自由基类型和限制的阻碍。肿瘤保留性能较差。在此,受具有 100% 活性药物成分 (API) 含量的全 API 纳米药物 (FAND) 新概念的启发,并将临床抗疟疾药物青蒿琥酯 (ARTE) 重新用作抗癌自由基发生器,AFeI FANDs 由ARTE、人体必需的 Fe3+ 和 FDA 批准的用于缺氧肿瘤治疗的荧光剂 ICG 均经过合理设计和构建。有吸引力的是,完全药物活性成分ARTE、Fe3+和ICG可以在酸性肿瘤微环境中响应性释放,并协同产生五种类型的自由基,包括·O2−、·C、·OH、LOO·和1O2,从而产生强大的线粒体损伤、核 DNA 损伤和脂质过氧化物。更重要的是,AFeI FAND 在轻度近红外辐射下表现出超过 108 小时的超长肿瘤保留和良好的肿瘤抑制结果。总的来说,所提出的基于 FAND 的五重自由基疗法的第一个范例为临床可转移的肿瘤治疗纳米药物的开发拓展了新的视野。











































 京公网安备 11010802027423号
京公网安备 11010802027423号