当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The sequential migration of rare earth elements (REE) in WE43 magnesium alloy during anodic oxidation treatment
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2024-06-18 , DOI: 10.1016/j.colsurfa.2024.134562 Xugang Lu , Siqi Zhang , Jun Chen , Mei Zhang , Yifan Cui , Yipu Cao , Shibing Xiong , Simeng Wang , Bangcheng Yang
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2024-06-18 , DOI: 10.1016/j.colsurfa.2024.134562 Xugang Lu , Siqi Zhang , Jun Chen , Mei Zhang , Yifan Cui , Yipu Cao , Shibing Xiong , Simeng Wang , Bangcheng Yang
|
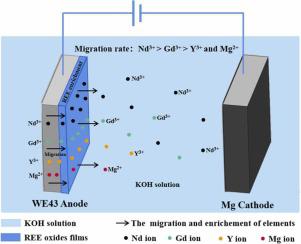
Rare earth elements (REE) like Y, Gd and Nd could migrate and enrich on WE43 magnesium alloy (WE43) surface during anodic oxidation. However, the specific mechanism is still unclear. In this study, WE43 alloy was modified by anodizing for 10 min, 30 min, 1 h and 2 h in KOH solution, respectively, followed by heating treatment for 2 h at 500℃. The obtained materials were named as AO-10, AO-30, AO-1 h and AO-2 h in turn. The surface morphology, phase compositions, surface potentials, REE contents of these materials were studied. In addition, the contents of REE in materials and KOH solution were investigated before and after electrolysis. The results found that surface morphology, phase composition were different with different electrolytic time. The phase compositions changed from GdO and NdO on AO-10 surface to YO and GdO on AO-2 h surface. Moreover, The contents of REE on material surfaces would first increase and then decrease with over time. Surface potentials confirmed that the regions rich in REE had lower potentials than Mg substrate. The contents of REE in materials and KOH solution further revealed that Nd ion was the first to ionize into the solution, followed by Gd, finally by Y. The migration rates of REE were different, which depends on their electric potentials. The migration rate of Nd was the fastest in all elements. It would preferentially migrate to material surface and entered into electrolyte solution in the oxidation process. Hence, the migration of REE from the inside to the surface of WE43 metal is sequential during anodic oxidation.
中文翻译:

WE43镁合金阳极氧化处理过程中稀土元素的序贯迁移
Y、Gd、Nd等稀土元素在阳极氧化过程中会在WE43镁合金(WE43)表面迁移富集。但具体机制仍不清楚。本研究对WE43合金进行改性,分别在KOH溶液中阳极氧化10min、30min、1h和2h,然后在500℃下热处理2h。所得材料依次命名为AO-10、AO-30、AO-1h、AO-2h。研究了这些材料的表面形貌、物相组成、表面电位、稀土元素含量。此外,还考察了电解前后材料和KOH溶液中REE的含量。结果发现,不同电解时间的表面形貌、物相组成不同。相组成从 AO-10 表面上的 GdO 和 NdO 变为 AO-2h 表面上的 YO 和 GdO。而且,随着时间的推移,材料表面的REE含量先增加后减少。表面电位证实富含稀土元素的区域的电位低于镁基底的电位。材料和KOH溶液中REE的含量进一步表明,Nd离子首先电离到溶液中,其次是Gd,最后是Y。REE的迁移速率不同,这取决于它们的电势。 Nd的迁移速度是所有元素中最快的。在氧化过程中它会优先迁移到材料表面并进入电解质溶液。因此,在阳极氧化过程中,稀土元素从WE43金属内部到表面的迁移是连续的。
更新日期:2024-06-18
中文翻译:

WE43镁合金阳极氧化处理过程中稀土元素的序贯迁移
Y、Gd、Nd等稀土元素在阳极氧化过程中会在WE43镁合金(WE43)表面迁移富集。但具体机制仍不清楚。本研究对WE43合金进行改性,分别在KOH溶液中阳极氧化10min、30min、1h和2h,然后在500℃下热处理2h。所得材料依次命名为AO-10、AO-30、AO-1h、AO-2h。研究了这些材料的表面形貌、物相组成、表面电位、稀土元素含量。此外,还考察了电解前后材料和KOH溶液中REE的含量。结果发现,不同电解时间的表面形貌、物相组成不同。相组成从 AO-10 表面上的 GdO 和 NdO 变为 AO-2h 表面上的 YO 和 GdO。而且,随着时间的推移,材料表面的REE含量先增加后减少。表面电位证实富含稀土元素的区域的电位低于镁基底的电位。材料和KOH溶液中REE的含量进一步表明,Nd离子首先电离到溶液中,其次是Gd,最后是Y。REE的迁移速率不同,这取决于它们的电势。 Nd的迁移速度是所有元素中最快的。在氧化过程中它会优先迁移到材料表面并进入电解质溶液。因此,在阳极氧化过程中,稀土元素从WE43金属内部到表面的迁移是连续的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号