当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
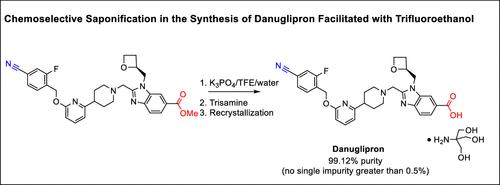
Chemoselective Saponification in the Synthesis of Danuglipron Facilitated with Trifluoroethanol
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-27 , DOI: 10.1021/acs.oprd.4c00085 Lu Han 1 , Bryan Li 1 , Ke Wang 1 , David B. Damon 1 , Javier Magano 1 , Mark T. Maloney 1 , Jason Mustakis 1 , Ronald J. Post 1 , Ruizhi Li 1 , Truong Nguyen 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-27 , DOI: 10.1021/acs.oprd.4c00085 Lu Han 1 , Bryan Li 1 , Ke Wang 1 , David B. Damon 1 , Javier Magano 1 , Mark T. Maloney 1 , Jason Mustakis 1 , Ronald J. Post 1 , Ruizhi Li 1 , Truong Nguyen 1
Affiliation
|
Danuglipron (PF-06882961), a potent, orally bioavailable small-molecule glucagon-like peptide-1 receptor (GLP-1R) agonist, is currently being developed for glycemic control among patients with Type-2 diabetes (J. Med. Chem. 2022, 65, 8208–8226; JAMA Netw. Open. 2023; 6(5): e2314493). The earlier synthesis of danuglipron suffered from chemoselective issues due to the competing nitrile hydrolysis in the final saponification step, which resulted in highly convoluted operations and extensive chromatographic purifications. We found that the methyl ester could be converted to trifluoroethyl ester, and the latter underwent hydrolysis to carboxylic acid in a much cleaner reaction profile. A thorough design of experiments (DOE) was conducted to expand the operating time window of the process to aid the process robustness during manufacturing. The improved process increased the yield by ∼20% and reduced the process mass intensity (PMI) by 86%.
中文翻译:

三氟乙醇促进化学选择性皂化合成 Danuglipron
Danuglipron (PF-06882961) 是一种有效的、口服生物可利用的小分子胰高血糖素样肽-1 受体 (GLP-1R) 激动剂,目前正在开发用于 2 型糖尿病患者的血糖控制 (J. Med. Chem. 2022, 65, 8208–8226; JAMA 网络 2023; 6(5): e2314493)。 Danuglipron 的早期合成由于最终皂化步骤中竞争性腈水解而存在化学选择性问题,导致高度复杂的操作和广泛的色谱纯化。我们发现甲酯可以转化为三氟乙酯,而后者以更干净的反应曲线水解为羧酸。进行了彻底的实验设计 (DOE),以扩大工艺的操作时间窗口,以帮助制造过程中工艺的稳健性。改进后的工艺将产量提高了约 20%,并将工艺质量强度 (PMI) 降低了 86%。
更新日期:2024-06-30
中文翻译:

三氟乙醇促进化学选择性皂化合成 Danuglipron
Danuglipron (PF-06882961) 是一种有效的、口服生物可利用的小分子胰高血糖素样肽-1 受体 (GLP-1R) 激动剂,目前正在开发用于 2 型糖尿病患者的血糖控制 (J. Med. Chem. 2022, 65, 8208–8226; JAMA 网络 2023; 6(5): e2314493)。 Danuglipron 的早期合成由于最终皂化步骤中竞争性腈水解而存在化学选择性问题,导致高度复杂的操作和广泛的色谱纯化。我们发现甲酯可以转化为三氟乙酯,而后者以更干净的反应曲线水解为羧酸。进行了彻底的实验设计 (DOE),以扩大工艺的操作时间窗口,以帮助制造过程中工艺的稳健性。改进后的工艺将产量提高了约 20%,并将工艺质量强度 (PMI) 降低了 86%。






































 京公网安备 11010802027423号
京公网安备 11010802027423号