当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical Generation of Hydroxide and Hydrogen Peroxide for Hydrolysis of Sulfuryl Fluoride Fumigant
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-06-30 , DOI: 10.1021/acs.jafc.4c00864 Cindy Weng 1 , Cade Napier 1 , Cedric Katte 1 , Spencer S. Walse 2 , William A. Mitch 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-06-30 , DOI: 10.1021/acs.jafc.4c00864 Cindy Weng 1 , Cade Napier 1 , Cedric Katte 1 , Spencer S. Walse 2 , William A. Mitch 1
Affiliation
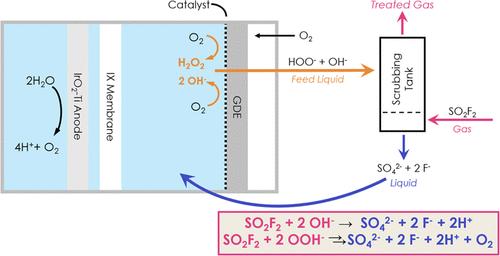
|
The post-harvest fumigant, sulfuryl fluoride (SO2F2), is a >1000-fold more potent greenhouse gas than carbon dioxide and methane. Pilot studies have shown that SO2F2 fumes vented from fumigation chambers can be captured and hydrolyzed by hydroxide (OH–) and hydrogen peroxide (H2O2) at pH ∼ 12 in a scrubber, producing SO42– and F– as waste salts. To reduce the costs and challenges associated with purchasing and mixing these reagents onsite, this study evaluates the electrochemical generation of OH– and H2O2 within spent scrubbing solution, taking advantage of the waste SO42– and F– as free sources of electrolyte. The study used a gas diffusion electrode constructed from carbon paper coated with carbon black as a catalyst selective for the reduction of O2 to H2O2. Under galvanostatic conditions, the study evaluated the effect of electrochemical conditions, including applied cathodic current density and electrolyte strength. Within an electrolyte containing 200 mM SO42– and 400 mM F–, comparable to the waste salts generated by a SO2F2 scrubbing event, the system produced 250 mM H2O2 at pH 12.6 within 4 h with a Faradaic efficiency of 98.8% for O2 reduction to H2O2. In a scrubbing-water sample from lab-scale fumigation, the system generated ∼200 mM H2O2 at pH 13.5 within 4 h with a Faradaic efficiency of 75.6%. A comparison of the costs to purchase NaOH and H2O2 against the electricity costs for electrochemical treatment indicated that the electrochemical approach could be 38–71% lower, depending on the local cost of electricity.
中文翻译:

电化学生成氢氧化物和过氧化氢用于硫酰氟熏蒸剂水解
收获后熏蒸剂硫酰氟 (SO 2 F 2 ) 是一种比二氧化碳和甲烷强 1000 倍以上的温室气体。试点研究表明,熏蒸室排出的SO 2 F 2 烟雾可被氢氧化物(OH – )和过氧化氢(H O 2 ),pH ∼ 12,在洗涤器中,产生 SO 4 2– 和 F – 作为废盐。为了降低现场购买和混合这些试剂的成本和挑战,本研究评估了废洗涤剂中 OH – 和 H 2 O 2 的电化学生成溶液,利用废 SO 4 2– 和 F – 作为电解质的免费来源。该研究使用由涂有炭黑的碳纸制成的气体扩散电极作为选择性将 O 2 还原为 H 2 O 2 的催化剂。在恒电流条件下,该研究评估了电化学条件的影响,包括施加的阴极电流密度和电解质强度。在含有 200 mM SO 4 2– 和 400 mM F – 的电解液中,与 SO 2 F 产生的废盐相当 2 洗涤事件,系统在 4 小时内产生 250 mM H 2 O 2 (pH 12.6),O 2 O 2 。在实验室规模熏蒸的洗涤水样品中,该系统在 4 小时内产生了约 200 mM H 2 O 2 (pH 13.5),法拉第效率为 75.6%。 将购买 NaOH 和 H 2 O 2 的成本与电化学处理的电力成本进行比较表明,电化学方法可以降低 38–71%,具体取决于当地成本的电力。
更新日期:2024-06-30
中文翻译:

电化学生成氢氧化物和过氧化氢用于硫酰氟熏蒸剂水解
收获后熏蒸剂硫酰氟 (SO 2 F 2 ) 是一种比二氧化碳和甲烷强 1000 倍以上的温室气体。试点研究表明,熏蒸室排出的SO 2 F 2 烟雾可被氢氧化物(OH – )和过氧化氢(H O 2 ),pH ∼ 12,在洗涤器中,产生 SO 4 2– 和 F – 作为废盐。为了降低现场购买和混合这些试剂的成本和挑战,本研究评估了废洗涤剂中 OH – 和 H 2 O 2 的电化学生成溶液,利用废 SO 4 2– 和 F – 作为电解质的免费来源。该研究使用由涂有炭黑的碳纸制成的气体扩散电极作为选择性将 O 2 还原为 H 2 O 2 的催化剂。在恒电流条件下,该研究评估了电化学条件的影响,包括施加的阴极电流密度和电解质强度。在含有 200 mM SO 4 2– 和 400 mM F – 的电解液中,与 SO 2 F 产生的废盐相当 2 洗涤事件,系统在 4 小时内产生 250 mM H 2 O 2 (pH 12.6),O 2 O 2 。在实验室规模熏蒸的洗涤水样品中,该系统在 4 小时内产生了约 200 mM H 2 O 2 (pH 13.5),法拉第效率为 75.6%。 将购买 NaOH 和 H 2 O 2 的成本与电化学处理的电力成本进行比较表明,电化学方法可以降低 38–71%,具体取决于当地成本的电力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号