当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Glomerulus-Targeted ROS-Responsive Polymeric Nanoparticles for Effective Membranous Nephropathy Therapy
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-06-28 , DOI: 10.1021/acsami.4c04345 Ling Guo 1 , Hanyu Yan 1 , Qinqin Gong 1 , Weili Zheng 1 , Liang Zhong 1 , Tao Gong 2 , Xun Sun 2 , Zhirong Zhang 2 , Yuan Ping 3 , Zilan Zhu 4 , Jian Xu 1 , Yongping Zhang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-06-28 , DOI: 10.1021/acsami.4c04345 Ling Guo 1 , Hanyu Yan 1 , Qinqin Gong 1 , Weili Zheng 1 , Liang Zhong 1 , Tao Gong 2 , Xun Sun 2 , Zhirong Zhang 2 , Yuan Ping 3 , Zilan Zhu 4 , Jian Xu 1 , Yongping Zhang 1
Affiliation
|
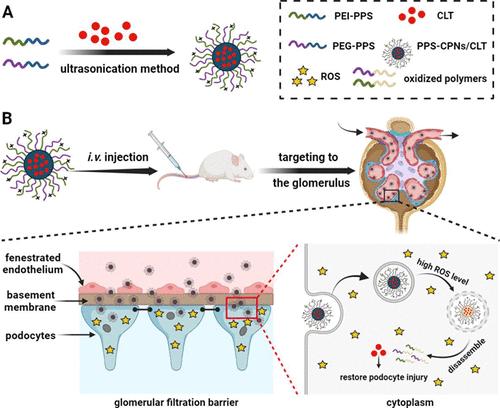
Membranous nephropathy (MN) is a common immune-mediated glomerular disease that requires the development of safe and highly effective therapies. Celastrol (CLT) has shown promise as a therapeutic molecule candidate, but its clinical use is currently limited due to off-target toxicity. Given that excess levels of reactive oxygen species (ROS) contributing to podocyte damage is a key driver of MN progression to end-stage renal disease, we rationally designed ROS-responsive cationic polymeric nanoparticles (PPS-CPNs) with a well-defined particle size and surface charge by employing poly(propylene sulfide)–polyethylene glycol (PPS–PEG) and poly(propylene sulfide)–polyethylenimine (PPS–PEI) to selectively deliver CLT to the damaged glomerulus for MN therapy. Experimental results show that PPS-CPNs successfully crossed the fenestrated endothelium, accumulated in the glomerular basement membrane (GBM), and were internalized by podocytes where rapid drug release was triggered by the overproduction of ROS, thereby outperforming nonresponsive CLT nanotherapy to alleviate subepithelial immune deposits, podocyte foot process effacement, and GBM expansion in a rat MN model. Moreover, the ROS-responsive CLT nanotherapy was associated with significantly lower toxicity to major organs than free CLT. These results suggest that encapsulating CLT into PPS-CPNs can improve efficacy and reduce toxicity as a promising treatment option for MN.
中文翻译:

肾小球靶向 ROS 响应性聚合物纳米颗粒可有效治疗膜性肾病
膜性肾病(MN)是一种常见的免疫介导的肾小球疾病,需要开发安全高效的治疗方法。雷公藤红素 (CLT) 作为一种候选治疗分子已显示出前景,但由于脱靶毒性,其临床应用目前受到限制。鉴于导致足细胞损伤的过量活性氧 (ROS) 是 MN 进展为终末期肾病的关键驱动因素,我们合理设计了具有明确粒径的 ROS 响应性阳离子聚合物纳米颗粒 (PPS-CPN)通过使用聚(丙烯硫醚)-聚乙二醇(PPS-PEG)和聚(丙烯硫醚)-聚乙烯亚胺(PPS-PEI)来选择性地将CLT递送至受损肾小球以进行MN治疗。实验结果表明,PPS-CPNs成功穿过有孔内皮,积聚在肾小球基底膜(GBM)中,并被足细胞内化,其中ROS的过量产生触发药物快速释放,从而优于无反应的CLT纳米疗法来减轻上皮下免疫沉积、大鼠 MN 模型中足细胞足突消失和 GBM 扩张。此外,与游离 CLT 相比,ROS 响应性 CLT 纳米疗法对主要器官的毒性显着降低。这些结果表明,将 CLT 封装到 PPS-CPN 中可以提高疗效并降低毒性,作为 MN 的一种有前途的治疗选择。
更新日期:2024-06-30
中文翻译:

肾小球靶向 ROS 响应性聚合物纳米颗粒可有效治疗膜性肾病
膜性肾病(MN)是一种常见的免疫介导的肾小球疾病,需要开发安全高效的治疗方法。雷公藤红素 (CLT) 作为一种候选治疗分子已显示出前景,但由于脱靶毒性,其临床应用目前受到限制。鉴于导致足细胞损伤的过量活性氧 (ROS) 是 MN 进展为终末期肾病的关键驱动因素,我们合理设计了具有明确粒径的 ROS 响应性阳离子聚合物纳米颗粒 (PPS-CPN)通过使用聚(丙烯硫醚)-聚乙二醇(PPS-PEG)和聚(丙烯硫醚)-聚乙烯亚胺(PPS-PEI)来选择性地将CLT递送至受损肾小球以进行MN治疗。实验结果表明,PPS-CPNs成功穿过有孔内皮,积聚在肾小球基底膜(GBM)中,并被足细胞内化,其中ROS的过量产生触发药物快速释放,从而优于无反应的CLT纳米疗法来减轻上皮下免疫沉积、大鼠 MN 模型中足细胞足突消失和 GBM 扩张。此外,与游离 CLT 相比,ROS 响应性 CLT 纳米疗法对主要器官的毒性显着降低。这些结果表明,将 CLT 封装到 PPS-CPN 中可以提高疗效并降低毒性,作为 MN 的一种有前途的治疗选择。






































 京公网安备 11010802027423号
京公网安备 11010802027423号