当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coupling Peptide-Based Encapsulation of Enzymes with Bacteria for Paraoxon Bioremediation
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-06-26 , DOI: 10.1021/acsami.4c06501 Yoav Dan 1, 2, 3 , David Gurevich 4 , Ofir Gershoni 4 , Francesca Netti 1, 2, 3 , Lihi Adler-Abramovich 1, 2, 3 , Livnat Afriat-Jurnou 4, 5
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-06-26 , DOI: 10.1021/acsami.4c06501 Yoav Dan 1, 2, 3 , David Gurevich 4 , Ofir Gershoni 4 , Francesca Netti 1, 2, 3 , Lihi Adler-Abramovich 1, 2, 3 , Livnat Afriat-Jurnou 4, 5
Affiliation
|
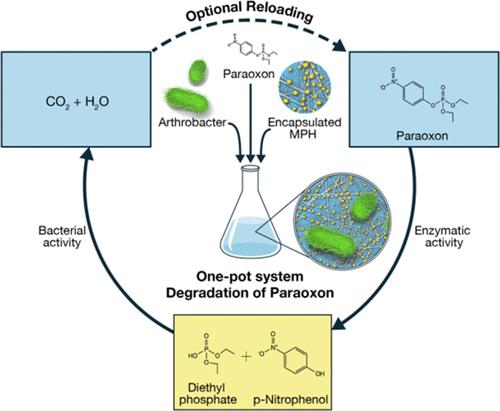
The catalytic efficiency of enzymes can be harnessed as an environmentally friendly solution for decontaminating various xenobiotics and toxins. However, for some xenobiotics, several enzymatic steps are needed to obtain nontoxic products. Another challenge is the low durability and stability of many native enzymes in their purified form. Herein, we coupled peptide-based encapsulation of bacterial phosphotriesterase with soil-originated bacteria, Arthrobacter sp. 4Hβ as an efficient system capable of biodegradation of paraoxon, a neurotoxin pesticide. Specifically, recombinantly expressed and purified methyl parathion hydrolase (MPH), with high hydrolytic activity toward paraoxon, was encapsulated within peptide nanofibrils, resulting in increased shelf life and retaining ∼50% activity after 132 days since purification. Next, the addition of Arthrobacter sp. 4Hβ, capable of degrading para-nitrophenol (PNP), the hydrolysis product of paraoxon, which is still toxic, resulted in nondetectable levels of PNP. These results present an efficient one-pot system that can be further developed as an environmentally friendly solution, coupling purified enzymes and native bacteria, for pesticide bioremediation. We further suggest that this system can be tailored for different xenobiotics by encapsulating the rate-limiting key enzymes followed by their combination with environmental bacteria that can use the enzymatic step products for full degradation without the need to engineer synthetic bacteria.
中文翻译:

基于肽的酶与细菌的偶联封装用于对氧磷生物修复
酶的催化效率可以作为一种环境友好的解决方案来净化各种异生物质和毒素。然而,对于某些外源物质,需要几个酶促步骤才能获得无毒产品。另一个挑战是许多纯化形式的天然酶的耐用性和稳定性较低。在此,我们将细菌磷酸三酯酶的基于肽的封装与土壤来源的细菌节杆菌结合起来。 4Hβ 是一种能够生物降解对氧磷(一种神经毒素农药)的有效系统。具体来说,重组表达和纯化的甲基对硫磷水解酶(MPH)对对氧磷具有高水解活性,被封装在肽纳米纤维内,从而延长了保质期,并在纯化后 132 天后保留了约 50% 的活性。接下来,添加节杆菌。 4Hβ能够降解对硝基苯酚(PNP),即对氧磷的水解产物,但仍然有毒,导致 PNP 水平不可检测。这些结果提出了一种高效的一锅系统,可以进一步开发为一种环境友好的解决方案,将纯化的酶和天然细菌结合起来,用于农药生物修复。我们进一步建议,该系统可以针对不同的外源物质进行定制,方法是封装限速关键酶,然后将其与环境细菌结合,从而可以利用酶促步骤产物进行完全降解,而无需设计合成细菌。
更新日期:2024-06-30
中文翻译:

基于肽的酶与细菌的偶联封装用于对氧磷生物修复
酶的催化效率可以作为一种环境友好的解决方案来净化各种异生物质和毒素。然而,对于某些外源物质,需要几个酶促步骤才能获得无毒产品。另一个挑战是许多纯化形式的天然酶的耐用性和稳定性较低。在此,我们将细菌磷酸三酯酶的基于肽的封装与土壤来源的细菌节杆菌结合起来。 4Hβ 是一种能够生物降解对氧磷(一种神经毒素农药)的有效系统。具体来说,重组表达和纯化的甲基对硫磷水解酶(MPH)对对氧磷具有高水解活性,被封装在肽纳米纤维内,从而延长了保质期,并在纯化后 132 天后保留了约 50% 的活性。接下来,添加节杆菌。 4Hβ能够降解对硝基苯酚(PNP),即对氧磷的水解产物,但仍然有毒,导致 PNP 水平不可检测。这些结果提出了一种高效的一锅系统,可以进一步开发为一种环境友好的解决方案,将纯化的酶和天然细菌结合起来,用于农药生物修复。我们进一步建议,该系统可以针对不同的外源物质进行定制,方法是封装限速关键酶,然后将其与环境细菌结合,从而可以利用酶促步骤产物进行完全降解,而无需设计合成细菌。






































 京公网安备 11010802027423号
京公网安备 11010802027423号