当前位置:
X-MOL 学术
›
Coord. Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The exotic quasisolidity and supersolidity of water
Coordination Chemistry Reviews ( IF 20.3 ) Pub Date : 2024-06-24 , DOI: 10.1016/j.ccr.2024.216042 Chang Q. Sun , Yong Zhou , Hengxin Fang , Sanmei Wang , Yongli Huang , Xi Zhang , Zengsheng Ma , Biao Wang
Coordination Chemistry Reviews ( IF 20.3 ) Pub Date : 2024-06-24 , DOI: 10.1016/j.ccr.2024.216042 Chang Q. Sun , Yong Zhou , Hengxin Fang , Sanmei Wang , Yongli Huang , Xi Zhang , Zengsheng Ma , Biao Wang
|
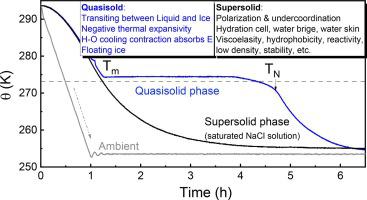
This work features the recent findings of the quasisolid (QS, or quasi-liquid) in the temperature domain and supersolid due to electric polarization or molecular undercoordination, which are beyond the scope of the P-T phase diagram. The cooperativity of the coupling O:H–O bond in its segmental length, energy, specific heat, and associated polarization derive these abnormal phases. The specific-heat disparity defines structure phases varying from XI to Vapor and mass density evolution under ambient pressure. Possessing negative thermal expansivity (NTE), the QS phase transfers the liquid to the solid phase, which fosters ice buoyancy. The supersolid phase presents in the skin of water, ice, and droplets, and the ionic hydration cells and volumetric water with a current flow or under ∼10 eV/cm electric bias. The H–O bond of the supersolid phase is 8 ∼ 10 % shorter and vibrating at frequencies 7.3 % higher than that of the standard pristine water (0.1 nm in length and 3250 cm in frequency). The supersolid has extraordinary elasticity, mechanical strength, optical reflectivity, structure order, thermal stability and diffusivity, catalytic capability, and chemical reactivity but 25 % lower mass density. The gel-like supersolidity endows ice and water with high hydrophobicity, viscoelasticity, catalytic and hydro-voltaic capability, and thermal diffusivity. The H–O bond contracts and absorbs energy during the Liquid-QS-Ice phase transition while doing contrastingly in the supersolid phase of the saturated NaCl solution under 254 K temperature.
中文翻译:

水的奇特的准固体和超固体
这项工作的特点是最近发现了温度域中的准固体(QS,或准液体)和由于电极化或分子配位不足而导致的超固体,这些超出了 P-T 相图的范围。耦合 O:H-O 键在其链段长度、能量、比热和相关极化方面的协同作用产生了这些异常相。比热差异定义了从 XI 到蒸气的结构相以及环境压力下的质量密度演变。 QS 相具有负热膨胀率 (NTE),可将液相转变为固相,从而增强冰的浮力。超固相存在于水、冰和水滴的表皮中,以及在电流流动或~10 eV/cm 电偏压下的离子水合细胞和体积水。超固相的H-O键比标准纯净水(长度0.1纳米,频率3250厘米)短8∼10%,振动频率高7.3%。超固体具有非凡的弹性、机械强度、光学反射率、结构有序性、热稳定性和扩散性、催化能力和化学反应性,但质量密度低 25%。凝胶状的超固体赋予冰和水高疏水性、粘弹性、催化和水伏能力以及热扩散性。 H-O 键在液体-QS-冰相变过程中收缩并吸收能量,而在 254 K 温度下饱和氯化钠溶液的超固相中则相反。
更新日期:2024-06-24
中文翻译:

水的奇特的准固体和超固体
这项工作的特点是最近发现了温度域中的准固体(QS,或准液体)和由于电极化或分子配位不足而导致的超固体,这些超出了 P-T 相图的范围。耦合 O:H-O 键在其链段长度、能量、比热和相关极化方面的协同作用产生了这些异常相。比热差异定义了从 XI 到蒸气的结构相以及环境压力下的质量密度演变。 QS 相具有负热膨胀率 (NTE),可将液相转变为固相,从而增强冰的浮力。超固相存在于水、冰和水滴的表皮中,以及在电流流动或~10 eV/cm 电偏压下的离子水合细胞和体积水。超固相的H-O键比标准纯净水(长度0.1纳米,频率3250厘米)短8∼10%,振动频率高7.3%。超固体具有非凡的弹性、机械强度、光学反射率、结构有序性、热稳定性和扩散性、催化能力和化学反应性,但质量密度低 25%。凝胶状的超固体赋予冰和水高疏水性、粘弹性、催化和水伏能力以及热扩散性。 H-O 键在液体-QS-冰相变过程中收缩并吸收能量,而在 254 K 温度下饱和氯化钠溶液的超固相中则相反。






































 京公网安备 11010802027423号
京公网安备 11010802027423号