当前位置:
X-MOL 学术
›
J. Mech. Phys. Solids
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanics and thermal analyses of microfluidic nerve-cooler system
Journal of the Mechanics and Physics of Solids ( IF 5.0 ) Pub Date : 2024-06-20 , DOI: 10.1016/j.jmps.2024.105741 Dongjun Bai , Zichen Zhao , Raudel Avila , Danli Xia , Yonggang Huang , John A. Rogers , Zhaoqian Xie
Journal of the Mechanics and Physics of Solids ( IF 5.0 ) Pub Date : 2024-06-20 , DOI: 10.1016/j.jmps.2024.105741 Dongjun Bai , Zichen Zhao , Raudel Avila , Danli Xia , Yonggang Huang , John A. Rogers , Zhaoqian Xie
|
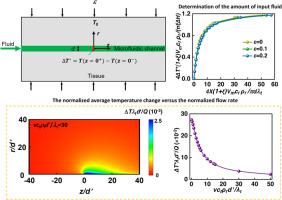
Recent advances in microelectronics and biomedical technology has led to capabilities for integrating programmable cooling mechanisms into bioelectronic devices for pain management and other important applications in human health. However, achieving localized and precise targeted cooling remains a challenge due to the influence of tissue mechanics during device placement/operation, viscous effects in the flow of coolants, and critical physical parameters that locally affect tissue temperature and mechanical responses. Recent studies demonstrate that bioelectronic devices can be equipped with microfluidic structures designed to support phase-change cooling mechanisms, with cooling power adjustable by control of fluid flow rates. When operated in closed-loop feedback schemes based on measurements of temperature at the tissue interface, these platforms can achieve precise temperature control of regions of peripheral nerves involved in pain signaling. Establishing a theoretical model to understand the effects of these devices on the underlying soft and deformable tissues, along with microfluidic deformations that can alter the heat transfer cooling process in viscous flow, is critical for optimized design and operation. To address this, a theoretical model is developed to describe the influence of remote strain in the tissue and to quantify the spatial temperature distribution of tissue cooled by fluid phase change in deformable microfluidic channels. A scaling law is derived to quantify the steady-state temperature distribution in the tissue under physiologically relevant mechanical loads, fluid viscosity, flow rates, and thermal conductivities, enabling the prediction of liquid and gas flux based on expected temperature changes and saturated vapor pressure in the deformed tissue and microchannel. These findings reveal that within a reasonable physiological range, the influence of fluid viscosity on temperature changes can be neglected, and the ratio of fluid to tissue thermal conductivity minimally impacts the temperature change at relatively high flow rates. Additionally, the model suggests a limit to tissue cooling for a given fluid, which may not necessarily increase with higher fluid volumes.
中文翻译:

微流控神经冷却器系统的力学和热分析
微电子和生物医学技术的最新进展使人们能够将可编程冷却机制集成到生物电子设备中,以用于疼痛管理和人类健康中的其他重要应用。然而,由于设备放置/操作过程中组织力学的影响、冷却剂流动中的粘性效应以及局部影响组织温度和机械响应的关键物理参数,实现局部和精确的目标冷却仍然是一个挑战。最近的研究表明,生物电子设备可以配备微流体结构,旨在支持相变冷却机制,通过控制流体流速来调节冷却功率。当基于组织界面温度测量的闭环反馈方案进行操作时,这些平台可以实现对参与疼痛信号传导的周围神经区域的精确温度控制。建立理论模型来了解这些装置对底层软组织和可变形组织的影响,以及可以改变粘性流中传热冷却过程的微流体变形,对于优化设计和操作至关重要。为了解决这个问题,开发了一个理论模型来描述组织中远程应变的影响,并量化由可变形微流体通道中的流体相变冷却的组织的空间温度分布。 推导出比例定律来量化在生理相关机械负载、流体粘度、流速和热导率下组织中的稳态温度分布,从而能够根据预期的温度变化和饱和蒸汽压来预测液体和气体通量。变形的组织和微通道。这些发现表明,在合理的生理范围内,流体粘度对温度变化的影响可以忽略不计,并且在相对高的流速下,流体与组织导热率的比率对温度变化的影响最小。此外,该模型还提出了给定液体的组织冷却的限制,该限制不一定会随着液体量的增加而增加。
更新日期:2024-06-20
中文翻译:

微流控神经冷却器系统的力学和热分析
微电子和生物医学技术的最新进展使人们能够将可编程冷却机制集成到生物电子设备中,以用于疼痛管理和人类健康中的其他重要应用。然而,由于设备放置/操作过程中组织力学的影响、冷却剂流动中的粘性效应以及局部影响组织温度和机械响应的关键物理参数,实现局部和精确的目标冷却仍然是一个挑战。最近的研究表明,生物电子设备可以配备微流体结构,旨在支持相变冷却机制,通过控制流体流速来调节冷却功率。当基于组织界面温度测量的闭环反馈方案进行操作时,这些平台可以实现对参与疼痛信号传导的周围神经区域的精确温度控制。建立理论模型来了解这些装置对底层软组织和可变形组织的影响,以及可以改变粘性流中传热冷却过程的微流体变形,对于优化设计和操作至关重要。为了解决这个问题,开发了一个理论模型来描述组织中远程应变的影响,并量化由可变形微流体通道中的流体相变冷却的组织的空间温度分布。 推导出比例定律来量化在生理相关机械负载、流体粘度、流速和热导率下组织中的稳态温度分布,从而能够根据预期的温度变化和饱和蒸汽压来预测液体和气体通量。变形的组织和微通道。这些发现表明,在合理的生理范围内,流体粘度对温度变化的影响可以忽略不计,并且在相对高的流速下,流体与组织导热率的比率对温度变化的影响最小。此外,该模型还提出了给定液体的组织冷却的限制,该限制不一定会随着液体量的增加而增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号