当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Impact of in vivo fate of STING agonist-loaded lipid nanoparticles on antitumor immunity
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-29 , DOI: 10.1016/j.jconrel.2024.06.064 Rikito Endo , Tomoki Ueda , Takumi Nagaoki , Natsumi Shima , Yusuke Sato , Hideyoshi Harashima , Takashi Nakamura
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-29 , DOI: 10.1016/j.jconrel.2024.06.064 Rikito Endo , Tomoki Ueda , Takumi Nagaoki , Natsumi Shima , Yusuke Sato , Hideyoshi Harashima , Takashi Nakamura
|
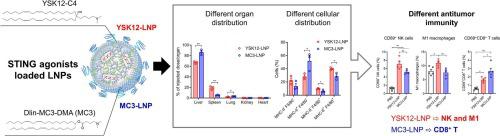
Therapeutically manipulating the stimulator of interferon genes (STING) pathway has promising potential for enhancing antitumor immunity. Agonists of this pathway (STING agonists) are being evaluated in clinical trials. Loading the STING agonists into lipid nanoparticles (LNPs) increases their safety and efficacy. We previously developed STING agonists loaded LNPs consisting of the ionizable lipid YSK12-C4 (YSK12-LNPs), which showed significant antitumor effects. However, it is largely unclear how the in vivo fate of STING agonists loaded LNPs affects the antitumor immune responses. In this study, we compared the YSK12-LNPs with LNPs composed of DLin-MC3-DMA (MC3-LNPs) showing different in vivo fates. Biodistribution and flow cytometry analyses of mouse tissues revealed that the MC3-LNPs delivered higher amounts of STING agonists to the liver than the YSK12-LNPs. Additionally, significantly more liver leukocytes internalized the MC3-LNPs than the YSK12-LNPs. In contrast, the YSK12-LNPs delivered higher amounts of STING agonists to the liver leukocytes than the MC3-LNPs, leading to the effective induction of innate immunity and inflammation in the tumors. However, the antitumor effects in the B16-F10 lung metastasis and CT26 tumor models were comparable. Interestingly, flow cytometry analyses suggested that the YSK12-LNPs were more likely to activate natural killer cells and M1 macrophages, while the MC3-LNPs were more likely to activate CD8 T cells. Our data suggest that different antitumor immune response mechanisms may operate depending on the characteristics and distribution of the LNPs.
中文翻译:

负载 STING 激动剂的脂质纳米粒子体内命运对抗肿瘤免疫的影响
治疗性操纵干扰素基因刺激物(STING)途径在增强抗肿瘤免疫力方面具有广阔的前景。该通路的激动剂(STING 激动剂)正在临床试验中进行评估。将 STING 激动剂装入脂质纳米颗粒 (LNP) 中可提高其安全性和有效性。我们之前开发了由可电离脂质 YSK12-C4 (YSK12-LNPs) 组成的负载 LNP 的 STING 激动剂,显示出显着的抗肿瘤作用。然而,目前尚不清楚负载 LNP 的 STING 激动剂的体内命运如何影响抗肿瘤免疫反应。在本研究中,我们比较了 YSK12-LNP 与由 DLin-MC3-DMA (MC3-LNP) 组成的 LNP,显示出不同的体内命运。小鼠组织的生物分布和流式细胞术分析表明,与 YSK12-LNP 相比,MC3-LNP 向肝脏输送的 STING 激动剂数量更高。此外,内化 MC3-LNP 的肝脏白细胞明显多于 YSK12-LNP。相比之下,YSK12-LNPs 比 MC3-LNPs 向肝脏白细胞递送更多量的 STING 激动剂,从而有效诱导肿瘤中的先天免疫和炎症。然而,B16-F10 肺转移和 CT26 肿瘤模型中的抗肿瘤效果相当。有趣的是,流式细胞术分析表明,YSK12-LNP 更有可能激活自然杀伤细胞和 M1 巨噬细胞,而 MC3-LNP 更有可能激活 CD8 T 细胞。我们的数据表明,不同的抗肿瘤免疫反应机制可能根据 LNP 的特征和分布而发挥作用。
更新日期:2024-06-29
中文翻译:

负载 STING 激动剂的脂质纳米粒子体内命运对抗肿瘤免疫的影响
治疗性操纵干扰素基因刺激物(STING)途径在增强抗肿瘤免疫力方面具有广阔的前景。该通路的激动剂(STING 激动剂)正在临床试验中进行评估。将 STING 激动剂装入脂质纳米颗粒 (LNP) 中可提高其安全性和有效性。我们之前开发了由可电离脂质 YSK12-C4 (YSK12-LNPs) 组成的负载 LNP 的 STING 激动剂,显示出显着的抗肿瘤作用。然而,目前尚不清楚负载 LNP 的 STING 激动剂的体内命运如何影响抗肿瘤免疫反应。在本研究中,我们比较了 YSK12-LNP 与由 DLin-MC3-DMA (MC3-LNP) 组成的 LNP,显示出不同的体内命运。小鼠组织的生物分布和流式细胞术分析表明,与 YSK12-LNP 相比,MC3-LNP 向肝脏输送的 STING 激动剂数量更高。此外,内化 MC3-LNP 的肝脏白细胞明显多于 YSK12-LNP。相比之下,YSK12-LNPs 比 MC3-LNPs 向肝脏白细胞递送更多量的 STING 激动剂,从而有效诱导肿瘤中的先天免疫和炎症。然而,B16-F10 肺转移和 CT26 肿瘤模型中的抗肿瘤效果相当。有趣的是,流式细胞术分析表明,YSK12-LNP 更有可能激活自然杀伤细胞和 M1 巨噬细胞,而 MC3-LNP 更有可能激活 CD8 T 细胞。我们的数据表明,不同的抗肿瘤免疫反应机制可能根据 LNP 的特征和分布而发挥作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号