当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biomimetic gold nanocages incorporating copper-human serum albumin for tumor immunotherapy via cuproptosis-lactate regulation
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-26 , DOI: 10.1016/j.jconrel.2024.06.059 Hajra Zafar , Jun Zhang , Faisal Raza , Xiuhua Pan , Zongwei Hu , Hanxiao Feng , Qi Shen
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-26 , DOI: 10.1016/j.jconrel.2024.06.059 Hajra Zafar , Jun Zhang , Faisal Raza , Xiuhua Pan , Zongwei Hu , Hanxiao Feng , Qi Shen
|
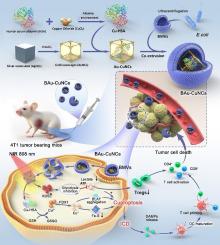
Cancer immunotherapy remains a significant challenge due to insufficient proliferation of immune cells and the sturdy immunosuppressive tumor microenvironment. Herein, we proposed the hypothesis of cuproptosis-lactate regulation to provoke cuproptosis and enhance anti-tumor immunity. For this purpose, copper-human serum albumin nanocomplex loaded gold nanocages with bacterial membrane coating (BAu-CuNCs) were developed. The targeted delivery and disassembly of BAu-CuNCs in tumor cells initiated a cascade of reactions. Under near infrared (NIR) laser irradiation, the release of copper-human serum albumin (Cu-HSA) was enhanced that reacted with intratumoral glutathione (GSH) via a disulfide exchange reaction to liberate Cu ions and exert cuproptosis. Subsequently, the cuproptosis effect triggered immunogenic cell death (ICD) in tumor by the release of damage associated molecular patterns (DAMPs) to realize anti-tumor immunity via robust production of cytotoxic T cells (CD8) and helper T cells (CD4). Meanwhile, under NIR irradiation, gold nanocages (AuNCs) promoted excessive reactive oxygen species (ROS) generation that played a primary role in inhibiting glycolysis, reducing the lactate and ATP level. The combine action of lower lactate level, ATP reduction and GSH depletion further sensitized the tumor cells to cuproptosis. Also, the lower lactate production led to the significant blockage of immunosuppressive T regulatory cells (Tregs) and boosted the anti-tumor immunity. Additionally, the effective inhibition of breast cancer metastasis to the lungs enhanced the anti-tumor therapeutic impact of BAu-CuNCs + NIR treatment. Hence, BAu-CuNCs + NIR concurrently induced cuproptosis, ICD and hindered lactate production, leading to the inhibition of tumor growth, remodeling of the immunosuppressive tumor microenvironment and suppression of lung metastasis. Therefore, leveraging cuproptosis-lactate regulation, this approach presents a novel strategy for enhanced tumor immunotherapy.
中文翻译:

结合铜-人血清白蛋白的仿生金纳米笼通过铜凋亡-乳酸调节进行肿瘤免疫治疗
由于免疫细胞增殖不足和强大的免疫抑制肿瘤微环境,癌症免疫治疗仍然是一个重大挑战。在此,我们提出了铜凋亡-乳酸调节的假说,以引发铜凋亡并增强抗肿瘤免疫力。为此,开发了带有细菌膜涂层的铜-人血清白蛋白纳米复合物负载金纳米笼(BAu-CuNC)。 BAu-CuNC 在肿瘤细胞中的靶向递送和分解引发了一系列反应。在近红外(NIR)激光照射下,铜-人血清白蛋白(Cu-HSA)的释放增强,与瘤内谷胱甘肽(GSH)通过二硫键交换反应释放铜离子并产生铜凋亡。随后,铜凋亡效应通过释放损伤相关分子模式(DAMP)触发肿瘤中的免疫原性细胞死亡(ICD),从而通过大量产生细胞毒性T细胞(CD8)和辅助性T细胞(CD4)来实现抗肿瘤免疫。同时,在近红外辐射下,金纳米笼(AuNC)促进了过量活性氧(ROS)的产生,这在抑制糖酵解、降低乳酸和ATP水平方面发挥了主要作用。乳酸水平降低、ATP 减少和 GSH 消耗的综合作用进一步使肿瘤细胞对铜凋亡更加敏感。此外,较低的乳酸产量会导致免疫抑制性 T 调节细胞 (Treg) 的显着阻断,从而增强抗肿瘤免疫力。此外,有效抑制乳腺癌肺部转移增强了 BAu-CuNCs + NIR 治疗的抗肿瘤治疗效果。 因此,BAu-CuNCs + NIR 同时诱导铜凋亡、ICD 并阻碍乳酸产生,从而抑制肿瘤生长、重塑免疫抑制性肿瘤微环境并抑制肺转移。因此,利用铜凋亡-乳酸调节,该方法提出了增强肿瘤免疫治疗的新策略。
更新日期:2024-06-26
中文翻译:

结合铜-人血清白蛋白的仿生金纳米笼通过铜凋亡-乳酸调节进行肿瘤免疫治疗
由于免疫细胞增殖不足和强大的免疫抑制肿瘤微环境,癌症免疫治疗仍然是一个重大挑战。在此,我们提出了铜凋亡-乳酸调节的假说,以引发铜凋亡并增强抗肿瘤免疫力。为此,开发了带有细菌膜涂层的铜-人血清白蛋白纳米复合物负载金纳米笼(BAu-CuNC)。 BAu-CuNC 在肿瘤细胞中的靶向递送和分解引发了一系列反应。在近红外(NIR)激光照射下,铜-人血清白蛋白(Cu-HSA)的释放增强,与瘤内谷胱甘肽(GSH)通过二硫键交换反应释放铜离子并产生铜凋亡。随后,铜凋亡效应通过释放损伤相关分子模式(DAMP)触发肿瘤中的免疫原性细胞死亡(ICD),从而通过大量产生细胞毒性T细胞(CD8)和辅助性T细胞(CD4)来实现抗肿瘤免疫。同时,在近红外辐射下,金纳米笼(AuNC)促进了过量活性氧(ROS)的产生,这在抑制糖酵解、降低乳酸和ATP水平方面发挥了主要作用。乳酸水平降低、ATP 减少和 GSH 消耗的综合作用进一步使肿瘤细胞对铜凋亡更加敏感。此外,较低的乳酸产量会导致免疫抑制性 T 调节细胞 (Treg) 的显着阻断,从而增强抗肿瘤免疫力。此外,有效抑制乳腺癌肺部转移增强了 BAu-CuNCs + NIR 治疗的抗肿瘤治疗效果。 因此,BAu-CuNCs + NIR 同时诱导铜凋亡、ICD 并阻碍乳酸产生,从而抑制肿瘤生长、重塑免疫抑制性肿瘤微环境并抑制肺转移。因此,利用铜凋亡-乳酸调节,该方法提出了增强肿瘤免疫治疗的新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号