当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Towards in vitro – In vivo correlation models for in situ forming drug implants
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-29 , DOI: 10.1016/j.jconrel.2024.06.058 Xiaoyi Wang , Mckenzie Roy , Ruifeng Wang , Owen Kwok , Yinhang Wang , Yan Wang , Bin Qin , Diane J. Burgess
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-29 , DOI: 10.1016/j.jconrel.2024.06.058 Xiaoyi Wang , Mckenzie Roy , Ruifeng Wang , Owen Kwok , Yinhang Wang , Yan Wang , Bin Qin , Diane J. Burgess
|
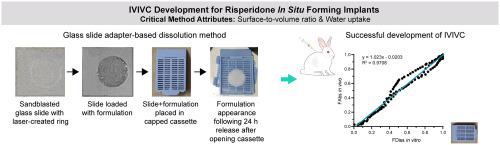
correlation (IVIVC) is a main focus of the pharmaceutical industry, academia and the regulatory sectors, as this is an effective modelling tool to predict drug product performance based on release data and serve as a surrogate for bioequivalence studies, significantly reducing the need for clinical studies. Till now, IVIVCs have not been successfully developed for forming implants due to the significantly different and drug release profiles that are typically achieved for these dosage forms. This is not unexpected considering the unique complexity of the drug release mechanisms of these products. Using risperidone forming implants as a model, the current work focuses on: identification of critical attributes of release testing methods that may contribute to differences in and drug release from forming implants; and optimization of the release method, with the aim of developing Level A IVIVCs for risperidone implants. Dissolution methods based on a novel Teflon shape controlling adapter along with a water non-dissolvable glass fiber membrane (GF/F) instead of a water dissolvable PVA film (named as GF/F-Teflon adapter and PVA-Teflon adapter, respectively), and an in-house fabricated Glass slide adapter were used to investigate the impact of: the surface-to-volume ratio, water uptake ratio, phase separation rate (measured by NMP release in 24 h post injection or ), and mechanical pressure on the drug release patterns. The surface-to-volume ratio and water uptake were shown to be more critical release testing method attributes compared to the phase separation rate and mechanical pressure. The Glass slide adapter-based dissolution method, which allowed for the formation of depots with bio-mimicking surface-to-volume ratios and sufficient water uptake, has the ability to generate bio-relevant degradation profiles as well as release profiles for risperidone implants. For the first time, a Level A IVIVC (rabbit model) has been successfully developed for forming implants. Release data for implant formulations with slightly different PLGA molecular weights (MWs) were used to develop the IVIVC. The predictability of the model passed external validation using the reference listed drug (RLD), Perseris®. IVIVC could not be developed when formulations with different PLGA molar ratios of lactic acid to glycolic acid (L/G) were included. The present work provides a comprehensive understanding of the impact of the testing method attributes on drug release from forming implants, which is a valuable practice for level A IVIVC development.
中文翻译:

迈向体外 - 原位形成药物植入物的体内相关模型
相关性(IVIVC)是制药行业、学术界和监管部门的主要关注点,因为这是一种有效的建模工具,可以根据释放数据预测药品性能,并作为生物等效性研究的替代品,从而显着减少临床需求学习。到目前为止,IVIVC 尚未成功开发用于形成植入物,因为这些剂型通常实现的药物释放曲线显着不同。考虑到这些产品药物释放机制的独特复杂性,这并不意外。使用利培酮成型植入物作为模型,目前的工作重点是: 确定释放测试方法的关键属性,这些属性可能导致成型植入物的药物释放差异;并优化释放方法,旨在开发利培酮植入剂的 A 级 IVIVC。基于新型聚四氟乙烯形状控制适配器以及不溶于水的玻璃纤维膜(GF/F)代替水溶性PVA膜(分别命名为GF/F-Teflon适配器和PVA-Teflon适配器)的溶解方法,和内部制造的载玻片适配器用于研究以下因素的影响:表面与体积比、吸水率、相分离率(通过注射后 24 小时内的 NMP 释放来测量或)以及机械压力对药物释放模式。与相分离速率和机械压力相比,表面积与体积比和吸水率被证明是更关键的释放测试方法属性。 基于玻璃载玻片适配器的溶解方法可以形成具有仿生表面与体积比和足够吸水性的储库,能够生成利培酮植入物的生物相关降解曲线和释放曲线。首次成功开发出 A 级 IVIVC(兔子模型),用于形成种植体。 PLGA 分子量 (MW) 略有不同的植入物配方的发布数据用于开发 IVIVC。该模型的可预测性通过了使用参比药物 (RLD) Perseris® 的外部验证。当包含具有不同乳酸与乙醇酸 (L/G) 摩尔比的 PLGA 制剂时,IVIVC 无法开发。目前的工作提供了对测试方法属性对成型植入物药物释放的影响的全面了解,这对于 A 级 IVIVC 开发来说是一个有价值的实践。
更新日期:2024-06-29
中文翻译:

迈向体外 - 原位形成药物植入物的体内相关模型
相关性(IVIVC)是制药行业、学术界和监管部门的主要关注点,因为这是一种有效的建模工具,可以根据释放数据预测药品性能,并作为生物等效性研究的替代品,从而显着减少临床需求学习。到目前为止,IVIVC 尚未成功开发用于形成植入物,因为这些剂型通常实现的药物释放曲线显着不同。考虑到这些产品药物释放机制的独特复杂性,这并不意外。使用利培酮成型植入物作为模型,目前的工作重点是: 确定释放测试方法的关键属性,这些属性可能导致成型植入物的药物释放差异;并优化释放方法,旨在开发利培酮植入剂的 A 级 IVIVC。基于新型聚四氟乙烯形状控制适配器以及不溶于水的玻璃纤维膜(GF/F)代替水溶性PVA膜(分别命名为GF/F-Teflon适配器和PVA-Teflon适配器)的溶解方法,和内部制造的载玻片适配器用于研究以下因素的影响:表面与体积比、吸水率、相分离率(通过注射后 24 小时内的 NMP 释放来测量或)以及机械压力对药物释放模式。与相分离速率和机械压力相比,表面积与体积比和吸水率被证明是更关键的释放测试方法属性。 基于玻璃载玻片适配器的溶解方法可以形成具有仿生表面与体积比和足够吸水性的储库,能够生成利培酮植入物的生物相关降解曲线和释放曲线。首次成功开发出 A 级 IVIVC(兔子模型),用于形成种植体。 PLGA 分子量 (MW) 略有不同的植入物配方的发布数据用于开发 IVIVC。该模型的可预测性通过了使用参比药物 (RLD) Perseris® 的外部验证。当包含具有不同乳酸与乙醇酸 (L/G) 摩尔比的 PLGA 制剂时,IVIVC 无法开发。目前的工作提供了对测试方法属性对成型植入物药物释放的影响的全面了解,这对于 A 级 IVIVC 开发来说是一个有价值的实践。











































 京公网安备 11010802027423号
京公网安备 11010802027423号