当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Manganese-induced Photothermal-Ferroptosis for Synergistic Tumor Therapy
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-25 , DOI: 10.1016/j.jconrel.2024.06.053 Kun Chen 1 , Rui Sun 2 , Yudong Guan 3 , Tao Fang 1 , Jun Tao 4 , Zhijie Li 3 , Bingchen Zhang 5 , Zhiqiang Yu 5 , Jiahang Tian 1 , Zhaogang Teng 4 , Jigang Wang 6
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-25 , DOI: 10.1016/j.jconrel.2024.06.053 Kun Chen 1 , Rui Sun 2 , Yudong Guan 3 , Tao Fang 1 , Jun Tao 4 , Zhijie Li 3 , Bingchen Zhang 5 , Zhiqiang Yu 5 , Jiahang Tian 1 , Zhaogang Teng 4 , Jigang Wang 6
Affiliation
|
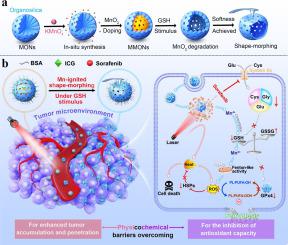
Ferroptosis-related tumor therapy based on nanomedicines has recently gained significant attention. However, the therapeutic performance is still hindered by the tumor's physical barriers such as the fibrotic tumor matrix and elevated interstitial fluid pressure, as well as chemical barriers like glutathione (GSH) overabundance. These physicochemical barriers impede the bioavailability of nanomedicines and compromise the therapeutic efficacy of lipid reactive oxygen species (ROS). Thus, this study pioneers a manganese-mediated overcoming of physicochemical barriers in the tumor microenvironment using organosilica-based nanomedicine (MMONs), which bolsters the synergy of photothermal-ferroptosis treatment. The MMONs display commendable proficiency in overcoming tumor physical barriers, due to their MnO-mediated shape-morphing and softness-transformation ability, which facilitates augmented cellular internalization, enhanced tumor accumulation, and superior drug penetration. Also, the MMONs possess excellent capability in chemical barrier overcoming, including MnO-mediated dual GSH clearance and enhanced ROS generation, which facilitates ferroptosis and heat shock protein inhibition. Notably, the resulting integration of physical and chemical barrier overcoming leads to amplified photothermal-ferroptosis synergistic tumor therapy both and . Accordingly, the comparative proteomic analysis has identified promoted ferroptosis with a transient inhibitory response observed in the mitochondria. This research aims to improve treatment strategies to better fight the complex defenses of tumors.
中文翻译:

锰诱导的光热铁死亡用于协同肿瘤治疗
基于纳米药物的铁死亡相关肿瘤治疗最近引起了广泛关注。然而,治疗效果仍然受到肿瘤的物理屏障(例如纤维化肿瘤基质和升高的间质液压力)以及化学屏障(例如谷胱甘肽(GSH)过多)的阻碍。这些物理化学障碍阻碍了纳米药物的生物利用度,并损害了脂质活性氧(ROS)的治疗功效。因此,这项研究开创了利用有机硅纳米药物(MMON)以锰介导克服肿瘤微环境中的物理化学障碍,从而增强了光热铁死亡治疗的协同作用。 MMONs 在克服肿瘤物理障碍方面表现出值得称赞的能力,因为它们具有 MnO 介导的形状变形和柔软度转变能力,有利于增强细胞内化、增强肿瘤积累和卓越的药物渗透。此外,MMONs 具有优异的克服化学屏障的能力,包括 MnO 介导的双重 GSH 清除和增强的 ROS 生成,从而促进铁死亡和热休克蛋白抑制。值得注意的是,克服物理和化学屏障的整合导致了光热-铁死亡协同肿瘤治疗的放大。因此,比较蛋白质组分析已鉴定出促进铁死亡并在线粒体中观察到短暂的抑制反应。这项研究旨在改进治疗策略,以更好地对抗肿瘤的复杂防御。
更新日期:2024-06-25
中文翻译:

锰诱导的光热铁死亡用于协同肿瘤治疗
基于纳米药物的铁死亡相关肿瘤治疗最近引起了广泛关注。然而,治疗效果仍然受到肿瘤的物理屏障(例如纤维化肿瘤基质和升高的间质液压力)以及化学屏障(例如谷胱甘肽(GSH)过多)的阻碍。这些物理化学障碍阻碍了纳米药物的生物利用度,并损害了脂质活性氧(ROS)的治疗功效。因此,这项研究开创了利用有机硅纳米药物(MMON)以锰介导克服肿瘤微环境中的物理化学障碍,从而增强了光热铁死亡治疗的协同作用。 MMONs 在克服肿瘤物理障碍方面表现出值得称赞的能力,因为它们具有 MnO 介导的形状变形和柔软度转变能力,有利于增强细胞内化、增强肿瘤积累和卓越的药物渗透。此外,MMONs 具有优异的克服化学屏障的能力,包括 MnO 介导的双重 GSH 清除和增强的 ROS 生成,从而促进铁死亡和热休克蛋白抑制。值得注意的是,克服物理和化学屏障的整合导致了光热-铁死亡协同肿瘤治疗的放大。因此,比较蛋白质组分析已鉴定出促进铁死亡并在线粒体中观察到短暂的抑制反应。这项研究旨在改进治疗策略,以更好地对抗肿瘤的复杂防御。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号