当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Auto-loaded TRAIL-exosomes derived from induced neural stem cells for brain cancer therapy
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-26 , DOI: 10.1016/j.jconrel.2024.06.048 Xiaopei Zhang , Hannah Taylor , Alain Valdivia , Rajaneekar Dasari , Andrew Buckley , Emily Bonacquisti , Juliane Nguyen , Krishna Kanchi , David L. Corcoran , Laura E. Herring , Dennis A. Steindler , Albert Baldwin , Shawn Hingtgen , Andrew Benson Satterlee
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-26 , DOI: 10.1016/j.jconrel.2024.06.048 Xiaopei Zhang , Hannah Taylor , Alain Valdivia , Rajaneekar Dasari , Andrew Buckley , Emily Bonacquisti , Juliane Nguyen , Krishna Kanchi , David L. Corcoran , Laura E. Herring , Dennis A. Steindler , Albert Baldwin , Shawn Hingtgen , Andrew Benson Satterlee
|
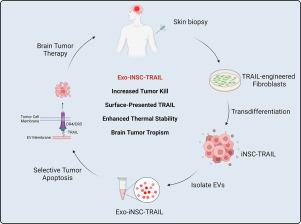
Transdifferentiation (TD), a somatic cell reprogramming process that eliminates pluripotent intermediates, creates cells that are ideal for personalized anti-cancer therapy. Here, we provide the first evidence that extracellular vesicles (EVs) from TD-derived induced neural stem cells (Exo-iNSCs) are an efficacious treatment strategy for brain cancer. We found that genetically engineered iNSCs generated EVs loaded with the tumoricidal gene product TRAIL at nearly twice the rate of their parental fibroblasts, and TRAIL produced by iNSCs was naturally loaded into the lumen of EVs and arrayed across their outer membrane (Exo-iNSC-TRAIL). Uptake studies in ex vivo organotypic brain slice cultures showed that Exo-iNSC-TRAIL selectively accumulates within tumor foci, and co-culture assays demonstrated that Exo-iNSC-TRAIL killed metastatic and primary brain cancer cells more effectively than free TRAIL. In an orthotopic mouse model of brain cancer, Exo-iNSC-TRAIL reduced breast-to-brain tumor xenografts by approximately 3000-fold compared to treatment with free TRAIL, with all Exo-iNSC-TRAIL treated animals surviving through 90 days post-treatment. In additional in vivo testing against aggressive U87 and invasive GBM8 glioblastoma tumors, Exo-iNSC-TRAIL also induced a statistically significant increase in survival. These studies establish a novel, easily generated, stable, tumor-targeted EV to efficaciously treat multiple forms of brain cancer.
中文翻译:

自动加载的源自诱导神经干细胞的 TRAIL 外泌体用于脑癌治疗
转分化 (TD) 是一种体细胞重编程过程,可消除多能中间体,创造出适合个性化抗癌治疗的细胞。在这里,我们提供了第一个证据,证明来自 TD 衍生的诱导神经干细胞 (Exo-iNSC) 的细胞外囊泡 (EV) 是脑癌的有效治疗策略。我们发现,基因工程 iNSC 生成的 EV 加载了杀肿瘤基因产物 TRAIL,其速度几乎是其亲代成纤维细胞的两倍,并且 iNSC 产生的 TRAIL 自然加载到 EV 的管腔中并排列在其外膜上(Exo-iNSC-TRAIL) )。离体器官型脑切片培养物的摄取研究表明,Exo-iNSC-TRAIL 选择性地在肿瘤病灶内积累,共培养测定表明,Exo-iNSC-TRAIL 比游离 TRAIL 更有效地杀死转移性和原发性脑癌细胞。在脑癌原位小鼠模型中,与使用游离 TRAIL 治疗相比,Exo-iNSC-TRAIL 使乳腺到脑部的肿瘤异种移植物减少了约 3000 倍,所有经过 Exo-iNSC-TRAIL 治疗的动物在治疗后都存活了 90 天。在针对侵袭性 U87 和侵袭性 GBM8 胶质母细胞瘤的其他体内测试中,Exo-iNSC-TRAIL 还诱导了具有统计学意义的存活率增加。这些研究建立了一种新型、易于生成、稳定的肿瘤靶向 EV,可有效治疗多种形式的脑癌。
更新日期:2024-06-26
中文翻译:

自动加载的源自诱导神经干细胞的 TRAIL 外泌体用于脑癌治疗
转分化 (TD) 是一种体细胞重编程过程,可消除多能中间体,创造出适合个性化抗癌治疗的细胞。在这里,我们提供了第一个证据,证明来自 TD 衍生的诱导神经干细胞 (Exo-iNSC) 的细胞外囊泡 (EV) 是脑癌的有效治疗策略。我们发现,基因工程 iNSC 生成的 EV 加载了杀肿瘤基因产物 TRAIL,其速度几乎是其亲代成纤维细胞的两倍,并且 iNSC 产生的 TRAIL 自然加载到 EV 的管腔中并排列在其外膜上(Exo-iNSC-TRAIL) )。离体器官型脑切片培养物的摄取研究表明,Exo-iNSC-TRAIL 选择性地在肿瘤病灶内积累,共培养测定表明,Exo-iNSC-TRAIL 比游离 TRAIL 更有效地杀死转移性和原发性脑癌细胞。在脑癌原位小鼠模型中,与使用游离 TRAIL 治疗相比,Exo-iNSC-TRAIL 使乳腺到脑部的肿瘤异种移植物减少了约 3000 倍,所有经过 Exo-iNSC-TRAIL 治疗的动物在治疗后都存活了 90 天。在针对侵袭性 U87 和侵袭性 GBM8 胶质母细胞瘤的其他体内测试中,Exo-iNSC-TRAIL 还诱导了具有统计学意义的存活率增加。这些研究建立了一种新型、易于生成、稳定的肿瘤靶向 EV,可有效治疗多种形式的脑癌。











































 京公网安备 11010802027423号
京公网安备 11010802027423号