当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
ROS-responsive biomimetic nanosystem camouflaged by hybrid membranes of platelet-exosomes engineered with neuronal targeting peptide for TBI therapy
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-28 , DOI: 10.1016/j.jconrel.2024.06.018 Yi Li , Xin Xin , Xun Zhou , Jingzhou Liu , Hangbing Liu , Shuo Yuan , Hanhan Liu , Wenyan Hao , Jiejie Sun , Yuli Wang , Wei Gong , Meiyan Yang , Zhiping Li , Yang Han , Chunsheng Gao , Yang Yang
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-28 , DOI: 10.1016/j.jconrel.2024.06.018 Yi Li , Xin Xin , Xun Zhou , Jingzhou Liu , Hangbing Liu , Shuo Yuan , Hanhan Liu , Wenyan Hao , Jiejie Sun , Yuli Wang , Wei Gong , Meiyan Yang , Zhiping Li , Yang Han , Chunsheng Gao , Yang Yang
|
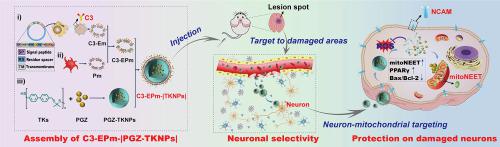
Recovery and survival following traumatic brain injury (TBI) depends on optimal amelioration of secondary injuries at lesion site. Delivering mitochondria-protecting drugs to neurons may revive damaged neurons at sites secondarily traumatized by TBI. Pioglitazone (PGZ) is a promising candidate for TBI treatment, limited by its low brain accumulation and poor targetability to neurons. Herein, we report a ROS-responsive nanosystem, camouflaged by hybrid membranes of platelets and engineered extracellular vesicles (EVs) (C3-EPm-|TKNPs|), that can be used for targeted delivery of PGZ for TBI therapy. Inspired by intrinsic ability of macrophages for inflammatory chemotaxis, engineered M2-like macrophage-derived EVs were constructed by fusing C3 peptide to EVs membrane integrator protein, Lamp2b, to confer them with ability to target neurons in inflamed lesions. Platelets provided hybridized EPm with capabilities to target hemorrhagic area caused by trauma via surface proteins. Consequently, C3-EPm-|PGZ-TKNPs| were orientedly delivered to neurons located in the traumatized hemisphere after intravenous administration, and triggered the release of PGZ from TKNPs via oxidative stress. The current work demonstrate that C3-EPm-|TKNPs| can effectively deliver PGZ to alleviate mitochondrial damage via mitoNEET for neuroprotection, further reversing behavioral deficits in TBI mice. Our findings provide proof-of-concept evidence of C3-EPm-|TKNPs|-derived nanodrugs as potential clinical approaches against neuroinflammation-related intracranial diseases.
中文翻译:

ROS响应仿生纳米系统,由血小板外泌体混合膜伪装,并用神经元靶向肽工程化,用于 TBI 治疗
创伤性脑损伤 (TBI) 后的恢复和生存取决于病变部位继发性损伤的最佳改善。向神经元输送线粒体保护药物可能会恢复因 TBI 二次损伤部位的受损神经元。吡格列酮 (PGZ) 是 TBI 治疗的一种有前途的候选药物,但由于其脑积聚率低且对神经元的靶向性差,因此受到限制。在此,我们报告了一种 ROS 响应性纳米系统,由血小板和工程化细胞外囊泡 (EV) (C3-EPm-|TKNPs|) 的混合膜伪装,可用于靶向递送 PGZ 用于 TBI 治疗。受巨噬细胞固有的炎症趋化能力的启发,通过将 C3 肽与 EV 膜整合蛋白 Lamp2b 融合,构建了工程化的 M2 样巨噬细胞衍生的 EV,赋予它们靶向发炎病变中神经元的能力。血小板提供杂交 EPm,能够通过表面蛋白靶向由创伤引起的出血区域。因此,C3-EPm-|PGZ-TKNPs|静脉注射后定向递送至位于受伤半球的神经元,并通过氧化应激触发TKNPs释放PGZ。目前的工作表明 C3-EPm-|TKNPs|可以有效地传递 PGZ,通过 mitoNEET 减轻线粒体损伤,实现神经保护,进一步逆转 TBI 小鼠的行为缺陷。我们的研究结果为 C3-EPm-|TKNPs| 衍生的纳米药物作为对抗神经炎症相关颅内疾病的潜在临床方法提供了概念验证证据。
更新日期:2024-06-28
中文翻译:

ROS响应仿生纳米系统,由血小板外泌体混合膜伪装,并用神经元靶向肽工程化,用于 TBI 治疗
创伤性脑损伤 (TBI) 后的恢复和生存取决于病变部位继发性损伤的最佳改善。向神经元输送线粒体保护药物可能会恢复因 TBI 二次损伤部位的受损神经元。吡格列酮 (PGZ) 是 TBI 治疗的一种有前途的候选药物,但由于其脑积聚率低且对神经元的靶向性差,因此受到限制。在此,我们报告了一种 ROS 响应性纳米系统,由血小板和工程化细胞外囊泡 (EV) (C3-EPm-|TKNPs|) 的混合膜伪装,可用于靶向递送 PGZ 用于 TBI 治疗。受巨噬细胞固有的炎症趋化能力的启发,通过将 C3 肽与 EV 膜整合蛋白 Lamp2b 融合,构建了工程化的 M2 样巨噬细胞衍生的 EV,赋予它们靶向发炎病变中神经元的能力。血小板提供杂交 EPm,能够通过表面蛋白靶向由创伤引起的出血区域。因此,C3-EPm-|PGZ-TKNPs|静脉注射后定向递送至位于受伤半球的神经元,并通过氧化应激触发TKNPs释放PGZ。目前的工作表明 C3-EPm-|TKNPs|可以有效地传递 PGZ,通过 mitoNEET 减轻线粒体损伤,实现神经保护,进一步逆转 TBI 小鼠的行为缺陷。我们的研究结果为 C3-EPm-|TKNPs| 衍生的纳米药物作为对抗神经炎症相关颅内疾病的潜在临床方法提供了概念验证证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号