当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Alendronate-functionalized porous nano-crystalsomes mitigate osteolysis and consequent inhibition of tumor growth in a tibia-induced metastasis model
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-25 , DOI: 10.1016/j.jconrel.2024.06.009 Ravi Prakash Shukla 1 , Pratiksha Tiwari 1 , Anirban Sardar 2 , Sandeep Urandur 1 , Shalini Gautam 1 , Disha Marwaha 1 , Ashish Kumar Tripathi 3 , Nikhil Rai 1 , Ritu Trivedi 2 , Prabhat Ranjan Mishra 4
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-25 , DOI: 10.1016/j.jconrel.2024.06.009 Ravi Prakash Shukla 1 , Pratiksha Tiwari 1 , Anirban Sardar 2 , Sandeep Urandur 1 , Shalini Gautam 1 , Disha Marwaha 1 , Ashish Kumar Tripathi 3 , Nikhil Rai 1 , Ritu Trivedi 2 , Prabhat Ranjan Mishra 4
Affiliation
|
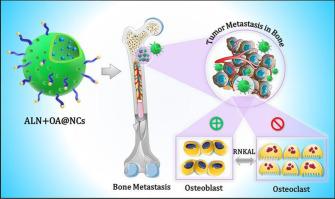
Bone is one of the most prevalent sites of metastases in various epithelial malignancies, including breast cancer and this metastasis to bone often leads to severe skeletal complications in women due to its osteolytic nature. To address this, we devised a novel drug delivery approach using an Alendronate (ALN) functionalized self-assembled porous crystalsomes for concurrent targeting of Oleanolic acid (OA) and ALN (ALN + OA@NCs) to bone metastasis. Initially, the conjugation of both PEG-OA and OA-PEG-ALN with ALN and OA was achieved, and this conjugation was then self-assembled into porous crystalsomes (ALN + OA@NCs) by nanoemulsion crystallization. The reconstruction of a 3D single particle using transmission electron microscopy ensured the crystalline porous structure of ALN + OA@NCs, was well aligned with characteristic nanoparticle attributes including size distribution, polydispersity, and zeta potential. Further, ALN + OA@NCs showed enhanced efficacy in comparison to OA@NCs suggesting the cytotoxic roles of ALN towards cancer cells, followed by augmentation ROS generation (40.81%), mitochondrial membrane depolarization (57.20%), and induction of apoptosis (40.43%). We found that ALN + OA@NCs facilitated inhibiting osteoclastogenesis and bone resorption followed by inhibited osteolysis. activity of ALN + OA@NCs in the 4 T1 cell-induced tibia model rendered a reduced bone loss in the treated mice followed by restoring bone morphometric markers which were further corroborated bone–targeting effects of ALN + OA@NCs to reduce RANKL-stimulated osteoclastogenesis. Further, intravenous pharmacokinetics showed the improved therapeutic profile of the ALN + OA@NCs in comparison to the free drug, prolonging the levels of the drug in the systemic compartment by reducing the clearance culminating the higher accumulation at the tumor site. Our finding proposed that ALN + OA@NCs can effectively target and treat breast cancer metastasis to bone and its associated complications.
中文翻译:

阿仑膦酸钠功能化多孔纳米晶体减轻胫骨诱导转移模型中的骨溶解并随后抑制肿瘤生长
骨是包括乳腺癌在内的各种上皮恶性肿瘤最常见的转移部位之一,由于其溶骨性质,这种骨转移常常导致女性严重的骨骼并发症。为了解决这个问题,我们设计了一种新型药物递送方法,使用阿仑膦酸钠(ALN)功能化自组装多孔晶体,同时靶向齐墩果酸(OA)和ALN(ALN + OA@NCs)骨转移。最初,实现了PEG-OA和OA-PEG-ALN与ALN和OA的缀合,然后通过纳米乳液结晶将这种缀合自组装成多孔晶体(ALN + OA@NCs)。使用透射电子显微镜重建 3D 单颗粒,确保 ALN + OA@NC 的晶体多孔结构与纳米颗粒特征属性(包括尺寸分布、多分散性和 zeta 电位)良好一致。此外,与 OA@NC 相比,ALN + OA@NC 的功效增强,表明 ALN 对癌细胞具有细胞毒作用,随后增加 ROS 生成 (40.81%)、线粒体膜去极化 (57.20%) 和诱导细胞凋亡 (40.43) %)。我们发现 ALN + OA@NCs 有助于抑制破骨细胞生成和骨吸收,进而抑制骨溶解。在 4 T1 细胞诱导的胫骨模型中,ALN + OA@NCs 的活性使治疗小鼠的骨丢失减少,随后恢复骨形态测量标记,这进一步证实了 ALN + OA@NCs 的骨靶向作用,以减少 RANKL 刺激破骨细胞生成。 此外,静脉内药代动力学显示,与游离药物相比,ALN + OA@NC 的治疗效果得到改善,通过减少清除率延长药物在全身区室中的水平,最终在肿瘤部位达到更高的积累。我们的研究结果表明,ALN + OA@NCs 可以有效靶向和治疗乳腺癌骨转移及其相关并发症。
更新日期:2024-06-25
中文翻译:

阿仑膦酸钠功能化多孔纳米晶体减轻胫骨诱导转移模型中的骨溶解并随后抑制肿瘤生长
骨是包括乳腺癌在内的各种上皮恶性肿瘤最常见的转移部位之一,由于其溶骨性质,这种骨转移常常导致女性严重的骨骼并发症。为了解决这个问题,我们设计了一种新型药物递送方法,使用阿仑膦酸钠(ALN)功能化自组装多孔晶体,同时靶向齐墩果酸(OA)和ALN(ALN + OA@NCs)骨转移。最初,实现了PEG-OA和OA-PEG-ALN与ALN和OA的缀合,然后通过纳米乳液结晶将这种缀合自组装成多孔晶体(ALN + OA@NCs)。使用透射电子显微镜重建 3D 单颗粒,确保 ALN + OA@NC 的晶体多孔结构与纳米颗粒特征属性(包括尺寸分布、多分散性和 zeta 电位)良好一致。此外,与 OA@NC 相比,ALN + OA@NC 的功效增强,表明 ALN 对癌细胞具有细胞毒作用,随后增加 ROS 生成 (40.81%)、线粒体膜去极化 (57.20%) 和诱导细胞凋亡 (40.43) %)。我们发现 ALN + OA@NCs 有助于抑制破骨细胞生成和骨吸收,进而抑制骨溶解。在 4 T1 细胞诱导的胫骨模型中,ALN + OA@NCs 的活性使治疗小鼠的骨丢失减少,随后恢复骨形态测量标记,这进一步证实了 ALN + OA@NCs 的骨靶向作用,以减少 RANKL 刺激破骨细胞生成。 此外,静脉内药代动力学显示,与游离药物相比,ALN + OA@NC 的治疗效果得到改善,通过减少清除率延长药物在全身区室中的水平,最终在肿瘤部位达到更高的积累。我们的研究结果表明,ALN + OA@NCs 可以有效靶向和治疗乳腺癌骨转移及其相关并发症。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号