当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of Surfactants on the Size Distribution and Electrocatalytic Nitrite Reduction of Uniformly Dispersed Au Nanoparticles
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-06-28 , DOI: 10.1021/acssuschemeng.3c07687 Wei Zhang 1 , Tianyi Wang 2 , Xiujing Xing 3 , Huhu Yin 1 , Jin Li 1 , Wei Xiong 1 , Hao Li 2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-06-28 , DOI: 10.1021/acssuschemeng.3c07687 Wei Zhang 1 , Tianyi Wang 2 , Xiujing Xing 3 , Huhu Yin 1 , Jin Li 1 , Wei Xiong 1 , Hao Li 2
Affiliation
|
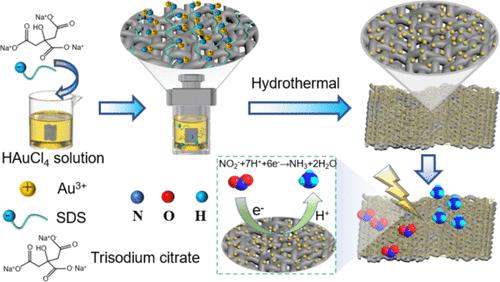
The electrocatalytic nitrite reduction reaction (NO2–RR) occurs under mild conditions at ambient temperature and pressure and can convert nitrite pollution into recycled ammonia. This method has great application potential in the purification of the water environment and the field of synthetic ammonia. In this article, we present a method to synthesize relatively uniformly dispersed gold nanoparticles on the surface of carbon cloth fibers (Au@CC) by hydrothermal self-assembly growth and calcination without the use of adhesive in the process. We studied the influence of different surfactants on the distribution and particle size of gold nanoparticles on carbon cloth by mechanism study and explored their effects on NO2–RR performance. The results showed that Au@CC with gold nanoparticles relatively uniformly dispersed on the surface of carbon cloth fibers and small particle size (≈86.2 nm) could be obtained when the sample was synthesized by the surfactant sodium dodecyl sulfate (SDS). This is mainly due to the strong coordination between SDS and the gold precursor and that the hydrophobic group of SDS can interact with the surface carbon cloth. When performed in the 0.1 M phosphate buffered saline (PBS) (with 0.1 M NaNO2) solution, the Au@CC-SDS electrode gave a high NH3 yield of 1249.1 μg h–1 cm–2 and faradaic efficiency of 80.7%. The density functional theory (DFT) calculations clarified the electrocatalytic reaction mechanism of the NO2–RR to NH3 on the Au active site. Thus, this study demonstrated that SDS is beneficial to the formation of relatively uniformly dispersed gold nanoparticles supported on carbon fiber cloth (Au@CC) and proved that gold-based nanomaterials are a promising catalyst for electrocatalytic NO2–RR ammonia synthesis.
中文翻译:

表面活性剂对均匀分散的纳米金颗粒尺寸分布及电催化亚硝酸盐还原的影响
电催化亚硝酸盐还原反应(NO 2 – RR)在环境温度和压力的温和条件下发生,可以将亚硝酸盐污染转化为回收氨。该方法在水环境净化和合成氨领域具有巨大的应用潜力。在本文中,我们提出了一种在碳布纤维(Au@CC)表面通过水热自组装生长和煅烧合成相对均匀分散的金纳米颗粒的方法,过程中不使用粘合剂。我们通过机理研究研究了不同表面活性剂对碳布上纳米金颗粒的分布和粒径的影响,并探讨了它们对NO 2 – RR性能的影响。结果表明,采用表面活性剂十二烷基硫酸钠(SDS)合成样品,可以得到金纳米粒子相对均匀地分散在碳布纤维表面、粒径较小(约86.2 nm)的Au@CC。这主要是由于SDS与金前驱体之间的强配位作用以及SDS的疏水基团可以与表面碳布相互作用。当在 0.1 M 磷酸盐缓冲盐水 (PBS)(含 0.1 M NaNO 2 )溶液中进行时,Au@CC-SDS 电极产生了 1249.1 μg h 的高 NH 3 产率 –1 cm –2 法拉第效率为 80.7%。密度泛函理论(DFT)计算阐明了Au活性位点上NO 2 – RR到NH 3 的电催化反应机理。 因此,本研究表明,SDS有利于形成相对均匀分散的金纳米粒子负载在碳纤维布(Au@CC)上,并证明金基纳米材料是一种很有前景的电催化NO催化剂 2 < b12> RR氨合成。
更新日期:2024-06-29
中文翻译:

表面活性剂对均匀分散的纳米金颗粒尺寸分布及电催化亚硝酸盐还原的影响
电催化亚硝酸盐还原反应(NO 2 – RR)在环境温度和压力的温和条件下发生,可以将亚硝酸盐污染转化为回收氨。该方法在水环境净化和合成氨领域具有巨大的应用潜力。在本文中,我们提出了一种在碳布纤维(Au@CC)表面通过水热自组装生长和煅烧合成相对均匀分散的金纳米颗粒的方法,过程中不使用粘合剂。我们通过机理研究研究了不同表面活性剂对碳布上纳米金颗粒的分布和粒径的影响,并探讨了它们对NO 2 – RR性能的影响。结果表明,采用表面活性剂十二烷基硫酸钠(SDS)合成样品,可以得到金纳米粒子相对均匀地分散在碳布纤维表面、粒径较小(约86.2 nm)的Au@CC。这主要是由于SDS与金前驱体之间的强配位作用以及SDS的疏水基团可以与表面碳布相互作用。当在 0.1 M 磷酸盐缓冲盐水 (PBS)(含 0.1 M NaNO 2 )溶液中进行时,Au@CC-SDS 电极产生了 1249.1 μg h 的高 NH 3 产率 –1 cm –2 法拉第效率为 80.7%。密度泛函理论(DFT)计算阐明了Au活性位点上NO 2 – RR到NH 3 的电催化反应机理。 因此,本研究表明,SDS有利于形成相对均匀分散的金纳米粒子负载在碳纤维布(Au@CC)上,并证明金基纳米材料是一种很有前景的电催化NO催化剂 2 < b12> RR氨合成。






































 京公网安备 11010802027423号
京公网安备 11010802027423号