当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Direct Sulfonylation of Styrenes and Unactivated Aliphatic Alkenes with Sulfonyl Chlorides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-27 , DOI: 10.1021/acs.joc.4c00094 Wei-Hao Rao 1 , Ying-Ge Li 1 , Li Li Jiang 1 , Chang Gao 1 , Yi-Zhuo Wang 1 , Jia-Fan Liu 1 , Fu-Yu Zhou 1 , Guo-Dong Zou 1 , Xinhua Cao 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-27 , DOI: 10.1021/acs.joc.4c00094 Wei-Hao Rao 1 , Ying-Ge Li 1 , Li Li Jiang 1 , Chang Gao 1 , Yi-Zhuo Wang 1 , Jia-Fan Liu 1 , Fu-Yu Zhou 1 , Guo-Dong Zou 1 , Xinhua Cao 1
Affiliation
|
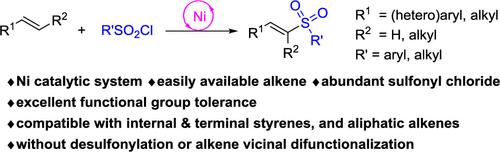
A nickel-catalyzed direct sulfonylation of alkenes with sulfonyl chlorides has been developed using 1,10-phenanthroline-5,6-dione as the ligand. Unactivated alkenes and styrenes including 1,1-, 1,2-disubstituted alkenes can be subjected to the protocol, and a wide range of vinyl sulfones was obtained in high to excellent yields with good functional group compatibility. Notably, the process did not allow the desulfonylation of sulfonyl chloride or chlorosulfonylation of alkenes. Radical-trapping experiment supported that a sulfonyl free-radical was likely produced and triggered subsequent transformation in the process.
中文翻译:

镍催化苯乙烯和未活化脂肪族烯烃与磺酰氯的直接磺酰化反应
使用 1,10-菲咯啉-5,6-二酮作为配体,开发了镍催化的烯烃与磺酰氯的直接磺酰化反应。未活化的烯烃和苯乙烯(包括 1,1-, 1,2-二取代烯烃)可以接受该方案,并且以高至优异的产率获得了各种乙烯基砜,并且具有良好的官能团相容性。值得注意的是,该方法不允许磺酰氯脱磺酰化或烯烃氯磺酰化。自由基捕获实验支持磺酰自由基可能产生并引发该过程中的后续转化。
更新日期:2024-06-27
中文翻译:

镍催化苯乙烯和未活化脂肪族烯烃与磺酰氯的直接磺酰化反应
使用 1,10-菲咯啉-5,6-二酮作为配体,开发了镍催化的烯烃与磺酰氯的直接磺酰化反应。未活化的烯烃和苯乙烯(包括 1,1-, 1,2-二取代烯烃)可以接受该方案,并且以高至优异的产率获得了各种乙烯基砜,并且具有良好的官能团相容性。值得注意的是,该方法不允许磺酰氯脱磺酰化或烯烃氯磺酰化。自由基捕获实验支持磺酰自由基可能产生并引发该过程中的后续转化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号