当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cobalt(II)-Substituted Cysteamine Dioxygenase Oxygenation Proceeds through a Cobalt(III)-Superoxo Complex
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-28 , DOI: 10.1021/jacs.4c01871 Jiasong Li 1 , Ran Duan 1 , Aimin Liu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-28 , DOI: 10.1021/jacs.4c01871 Jiasong Li 1 , Ran Duan 1 , Aimin Liu 1
Affiliation
|
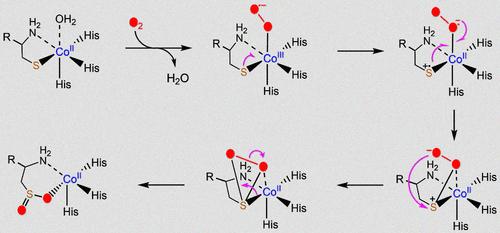
We investigated the metal-substituted catalytic activity of human cysteamine dioxygenase (ADO), an enzyme pivotal in regulating thiol metabolism and contributing to oxygen homeostasis. Our findings demonstrate the catalytic competence of cobalt(II)- and nickel(II)-substituted ADO in cysteamine oxygenation. Notably, Co(II)-ADO exhibited superiority over Ni(II)-ADO despite remaining significantly less active than the natural enzyme. Structural analyses through X-ray crystallography and cobalt K-edge excitation confirmed successful metal substitution with minimal structural perturbations. This provided a robust structural basis, supporting a conserved catalytic mechanism tailored to distinct metal centers. This finding challenges the proposed high-valent ferryl-based mechanism for thiol dioxygenases, suggesting a non-high-valent catalytic pathway in the native enzyme. Further investigation of the cysteamine-bound or a peptide mimic of N-terminus RGS5 bound Co(II)-ADO binary complex revealed the metal center’s high-spin (S = 3/2) state. Upon reaction with O2, a kinetically and spectroscopically detectable intermediate emerged with a ground spin state of S = 1/2. This intermediate exhibits a characteristic 59Co hyperfine splitting (A = 67 MHz) structure in the EPR spectrum alongside UV–vis features, consistent with known low-spin Co(III)-superoxo complexes. This observation, unique for protein-bound thiolate-ligated cobalt centers in a protein, unveils the capacities for O2 activation in such metal environments. These findings provide valuable insights into the non-heme iron-dependent thiol dioxygenase mechanistic landscape, furthering our understanding of thiol metabolism regulation. The exploration of metal-substituted ADO sheds light on the intricate interplay between metal and catalytic activity in this essential enzyme.
中文翻译:

钴 (II) 取代的半胱胺双加氧酶氧化作用通过钴 (III) - 超氧复合物进行
我们研究了人半胱胺双加氧酶 (ADO) 的金属取代催化活性,ADO 是调节硫醇代谢和促进氧稳态的关键酶。我们的研究结果证明了钴 (II) 和镍 (II) 取代的 ADO 在半胱胺氧化中的催化能力。值得注意的是,Co(II)-ADO 表现出优于 Ni(II)-ADO 的优势,尽管其活性明显低于天然酶。通过 X 射线晶体学和钴 K 边激发进行的结构分析证实了金属替代的成功,且结构扰动最小。这提供了强大的结构基础,支持针对不同金属中心定制的保守催化机制。这一发现挑战了所提出的硫醇双加氧酶的基于高价ferryl的机制,表明天然酶中存在非高价催化途径。对半胱胺结合或 N 末端 RGS5 结合的 Co(II)-ADO 二元复合物的肽模拟物的进一步研究揭示了金属中心的高自旋 (S = 3/2) 状态。与 O 2 反应后,出现了动力学和光谱可检测的中间体,其基自旋态为 S = 1/2。该中间体在 EPR 光谱中表现出特征性的 59 Co 超精细分裂 (A = 67 MHz) 结构以及 UV-vis 特征,与已知的低自旋 Co(III)-超氧配合物一致。这一观察结果对于蛋白质中与蛋白质结合的硫醇盐连接的钴中心来说是独一无二的,揭示了在此类金属环境中 O 2 活化的能力。这些发现为非血红素铁依赖性硫醇双加氧酶机制提供了宝贵的见解,进一步加深了我们对硫醇代谢调节的理解。 对金属取代的 ADO 的探索揭示了金属与这种必需酶催化活性之间复杂的相互作用。
更新日期:2024-06-28
中文翻译:

钴 (II) 取代的半胱胺双加氧酶氧化作用通过钴 (III) - 超氧复合物进行
我们研究了人半胱胺双加氧酶 (ADO) 的金属取代催化活性,ADO 是调节硫醇代谢和促进氧稳态的关键酶。我们的研究结果证明了钴 (II) 和镍 (II) 取代的 ADO 在半胱胺氧化中的催化能力。值得注意的是,Co(II)-ADO 表现出优于 Ni(II)-ADO 的优势,尽管其活性明显低于天然酶。通过 X 射线晶体学和钴 K 边激发进行的结构分析证实了金属替代的成功,且结构扰动最小。这提供了强大的结构基础,支持针对不同金属中心定制的保守催化机制。这一发现挑战了所提出的硫醇双加氧酶的基于高价ferryl的机制,表明天然酶中存在非高价催化途径。对半胱胺结合或 N 末端 RGS5 结合的 Co(II)-ADO 二元复合物的肽模拟物的进一步研究揭示了金属中心的高自旋 (S = 3/2) 状态。与 O 2 反应后,出现了动力学和光谱可检测的中间体,其基自旋态为 S = 1/2。该中间体在 EPR 光谱中表现出特征性的 59 Co 超精细分裂 (A = 67 MHz) 结构以及 UV-vis 特征,与已知的低自旋 Co(III)-超氧配合物一致。这一观察结果对于蛋白质中与蛋白质结合的硫醇盐连接的钴中心来说是独一无二的,揭示了在此类金属环境中 O 2 活化的能力。这些发现为非血红素铁依赖性硫醇双加氧酶机制提供了宝贵的见解,进一步加深了我们对硫醇代谢调节的理解。 对金属取代的 ADO 的探索揭示了金属与这种必需酶催化活性之间复杂的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号