当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Remote Functionalization by Pd-Catalyzed Isomerization of Alkynyl Alcohols
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-28 , DOI: 10.1021/jacs.4c05136 Simone Scaringi 1 , Baptiste Leforestier 1 , Clément Mazet 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-28 , DOI: 10.1021/jacs.4c05136 Simone Scaringi 1 , Baptiste Leforestier 1 , Clément Mazet 1
Affiliation
|
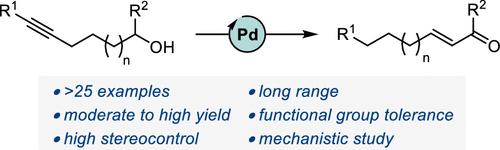
In recent years, progress has been made in the development of catalytic methods that allow remote functionalizations based on alkene isomerization. In contrast, protocols based on alkyne isomerization are comparatively rare. Herein, we report a general Pd-catalyzed long-range isomerization of alkynyl alcohols. Starting from aryl-, heteroaryl-, or alkyl-substituted precursors, the optimized system provides access preferentially to the thermodynamically more stable α,β-unsaturated aldehydes and is compatible with potentially sensitive functional groups. We showed that the migration of both π-components of the carbon–carbon triple bond can be sustained over several methylene units. Computational investigations served to shed light on the key elementary steps responsible for the reactivity and selectivity. These include an unorthodox phosphine-assisted deprotonation rather than a more conventional β-hydride elimination in the final tautomerization event.
中文翻译:

钯催化炔醇异构化的远程官能化
近年来,基于烯烃异构化的远程官能化催化方法的开发取得了进展。相比之下,基于炔烃异构化的方案相对较少。在此,我们报道了一种通用的 Pd 催化的炔醇长程异构化方法。从芳基、杂芳基或烷基取代的前体开始,优化的系统优先提供热力学更稳定的α,β-不饱和醛,并且与潜在敏感的官能团相容。我们证明碳-碳三键的两个 π 组分的迁移可以在几个亚甲基单元上持续。计算研究有助于阐明负责反应性和选择性的关键基本步骤。其中包括在最终互变异构事件中非正统的膦辅助去质子化而不是更传统的β-氢化物消除。
更新日期:2024-06-28
中文翻译:

钯催化炔醇异构化的远程官能化
近年来,基于烯烃异构化的远程官能化催化方法的开发取得了进展。相比之下,基于炔烃异构化的方案相对较少。在此,我们报道了一种通用的 Pd 催化的炔醇长程异构化方法。从芳基、杂芳基或烷基取代的前体开始,优化的系统优先提供热力学更稳定的α,β-不饱和醛,并且与潜在敏感的官能团相容。我们证明碳-碳三键的两个 π 组分的迁移可以在几个亚甲基单元上持续。计算研究有助于阐明负责反应性和选择性的关键基本步骤。其中包括在最终互变异构事件中非正统的膦辅助去质子化而不是更传统的β-氢化物消除。











































 京公网安备 11010802027423号
京公网安备 11010802027423号