当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational Studies of Reactions of 1,2,4,5-Tetrazines with Enamines in MeOH and HFIP
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-28 , DOI: 10.1021/jacs.4c06067 Pengchen Ma 1, 2 , Dennis Svatunek 2, 3 , Zixi Zhu 4 , Dale L. Boger 4 , Xin-Hua Duan 1 , K. N. Houk 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-28 , DOI: 10.1021/jacs.4c06067 Pengchen Ma 1, 2 , Dennis Svatunek 2, 3 , Zixi Zhu 4 , Dale L. Boger 4 , Xin-Hua Duan 1 , K. N. Houk 2
Affiliation
|
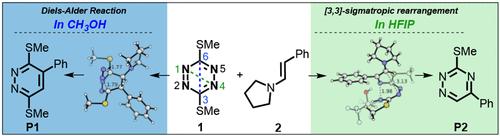
The reaction between 1,2,4,5-tetrazines and alkenes in polar solvents proceeds through a Diels–Alder cycloaddition along the C–C axis (C3/C6 cycloaddition) of the tetrazine, followed by dinitrogen loss. By contrast, the reactions of 1,2,4,5-tetrazines with enamines in hexafluoroisopropanol (HFIP) give 1,2,4-triazine products stemming from a formal Diels–Alder addition across the N–N axis (N1/N4 cycloaddition). We explored the mechanism of this interesting solvent effect through DFT calculations in detail and revealed a novel reaction pathway characterized by C–N bond formation, deprotonation, and a 3,3-sigmatropic rearrangement. The participation of an HFIP molecule was found to be crucial to the N1/N4 selectivity over C3/C6 due to the more favored initial C–N bond formation than C–C bond formation.
中文翻译:

1,2,4,5-四嗪与烯胺在 MeOH 和 HFIP 中反应的计算研究
1,2,4,5-四嗪和烯烃在极性溶剂中的反应通过沿四嗪的 C-C 轴(C3/C6 环加成)的 Diels-Alder 环加成反应进行,然后损失二氮。相比之下,1,2,4,5-四嗪与烯胺在六氟异丙醇 (HFIP) 中的反应产生 1,2,4-三嗪产物,该产物源自跨 N-N 轴的正式 Diels-Alder 加成(N1/N4 环加成) )。我们通过 DFT 计算详细探索了这种有趣的溶剂效应的机制,并揭示了一种以 C-N 键形成、去质子化和 3,3-σ 重排为特征的新反应途径。发现 HFIP 分子的参与对于 N1/N4 相对于 C3/C6 的选择性至关重要,因为初始 C-N 键形成比 C-C 键形成更有利。
更新日期:2024-06-28
中文翻译:

1,2,4,5-四嗪与烯胺在 MeOH 和 HFIP 中反应的计算研究
1,2,4,5-四嗪和烯烃在极性溶剂中的反应通过沿四嗪的 C-C 轴(C3/C6 环加成)的 Diels-Alder 环加成反应进行,然后损失二氮。相比之下,1,2,4,5-四嗪与烯胺在六氟异丙醇 (HFIP) 中的反应产生 1,2,4-三嗪产物,该产物源自跨 N-N 轴的正式 Diels-Alder 加成(N1/N4 环加成) )。我们通过 DFT 计算详细探索了这种有趣的溶剂效应的机制,并揭示了一种以 C-N 键形成、去质子化和 3,3-σ 重排为特征的新反应途径。发现 HFIP 分子的参与对于 N1/N4 相对于 C3/C6 的选择性至关重要,因为初始 C-N 键形成比 C-C 键形成更有利。











































 京公网安备 11010802027423号
京公网安备 11010802027423号