当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Hydroxylation of Tertiary Propargylic C(sp3)–H Bonds in Acyclic Systems: a Kinetic Resolution Study
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-27 , DOI: 10.1021/jacs.4c03610 Min Cao 1, 2 , Hongliang Wang 1, 2 , Fangao Hou 1 , Yuhang Zhu 1 , Qianqian Liu 1 , Chen-Ho Tung 1 , Lei Liu 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-27 , DOI: 10.1021/jacs.4c03610 Min Cao 1, 2 , Hongliang Wang 1, 2 , Fangao Hou 1 , Yuhang Zhu 1 , Qianqian Liu 1 , Chen-Ho Tung 1 , Lei Liu 1, 3
Affiliation
|
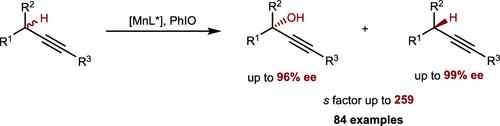
Direct site-selective and enantioselective oxyfunctionalization of C(sp3)–H bonds to form alcohols with a general scope, with predictable selectivities, and in preparatively useful yields represents a paradigm shift in the standard logic of synthetic organic chemistry. However, the knowledge of either enzymatic or nonenzymatic asymmetric hydroxylation of tertiary C–H bonds for enantioenriched tertiary alcohol synthesis is sorely lacking. Here, we report a practical manganese-catalyzed enantio-differentiating hydroxylation of tertiary propargylic C–H bonds in acyclic systems, producing a wide range of structurally diverse enantioenriched tertiary propargyl alcohols in high efficiency with extremely efficient chemo- and enantio-discrimination. Other features include the use of C–H substrates as the limiting reagent, noteworthy functional group compatibility, great synthetic utilities, and scalability. The findings serve as a blueprint for the development of metal-catalyzed asymmetric oxidation of challenging substrates.
中文翻译:

无环体系中叔丙炔 C(sp3)–H 键的催化对映选择性羟基化:动力学拆分研究
C(sp 3 )–H 键的直接位点选择性和对映选择性氧官能化形成具有一般范围、具有可预测选择性和制备有用产率的醇,代表了合成有机化学标准逻辑的范式转变。然而,对于用于对映体富集的叔醇合成的叔C-H键的酶促或非酶促不对称羟基化的了解非常缺乏。在这里,我们报道了无环体系中叔炔丙C-H键的实用锰催化对映差异化羟基化,以极其有效的化学和对映歧视的方式高效地产生了各种结构多样的对映体丰富的叔丙炔醇。其他特点包括使用 C-H 底物作为限制试剂、值得注意的官能团兼容性、出色的合成实用性和可扩展性。这些发现为开发具有挑战性的底物的金属催化不对称氧化提供了蓝图。
更新日期:2024-06-27
中文翻译:

无环体系中叔丙炔 C(sp3)–H 键的催化对映选择性羟基化:动力学拆分研究
C(sp 3 )–H 键的直接位点选择性和对映选择性氧官能化形成具有一般范围、具有可预测选择性和制备有用产率的醇,代表了合成有机化学标准逻辑的范式转变。然而,对于用于对映体富集的叔醇合成的叔C-H键的酶促或非酶促不对称羟基化的了解非常缺乏。在这里,我们报道了无环体系中叔炔丙C-H键的实用锰催化对映差异化羟基化,以极其有效的化学和对映歧视的方式高效地产生了各种结构多样的对映体丰富的叔丙炔醇。其他特点包括使用 C-H 底物作为限制试剂、值得注意的官能团兼容性、出色的合成实用性和可扩展性。这些发现为开发具有挑战性的底物的金属催化不对称氧化提供了蓝图。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号