当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electron Divergence of Cuδ− and Pdδ+ in Cu3Pd Alloy-Based Heterojunctions Boosts Concerted C≡C Bond Binding and the Volmer Step for Alkynol Semihydrogenation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-27 , DOI: 10.1021/jacs.4c03893 Xiu Lin 1 , Fan-Sheng Hu 1 , Qi-Yuan Li 1 , Dong Xu 1 , Yu-Shuai Xu 1 , Zhao Zhang 1 , Jie-Sheng Chen 1 , Xin-Hao Li 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-27 , DOI: 10.1021/jacs.4c03893 Xiu Lin 1 , Fan-Sheng Hu 1 , Qi-Yuan Li 1 , Dong Xu 1 , Yu-Shuai Xu 1 , Zhao Zhang 1 , Jie-Sheng Chen 1 , Xin-Hao Li 1
Affiliation
|
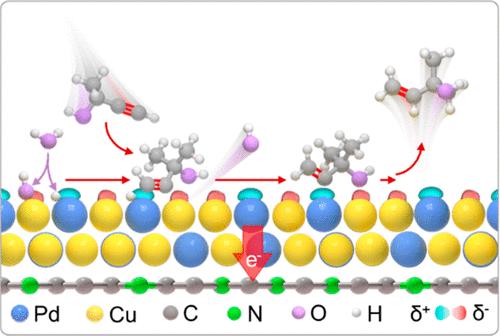
Electrocatalytic semihydrogenation of alkynols presents a sustainable alternative to conventional thermal methodologies for the high-value production of alkenols. The design of efficient catalysts with superior catalytic and energy efficiency for semihydrogenation poses a significant challenge. Here, we present the application of an electron-divergent Cu3Pd alloy-based heterojunction in promoting the electrocatalytic semihydrogenation of alkynols to alkenols using water as the proton source. The tunable electron divergence of Cuδ− and Pdδ+, modulated by rectifying contact with nitrogen-rich carbons, enables the concerted binding of active H species from the Volmer step of water dissociation and the C≡C bond of alkynols on Pdδ+ sites. Simultaneously, the pronounced electron divergence of Cu3Pd facilitates the universal adsorption of OH species from the Volmer step and alkynols on the Cuδ− sites. The electron-divergent dual-center substantially boosts water dissociation and inhibition of completing hydrogen evolution to give a turnover frequency of 2412 h–1, outperforming the reported electrocatalysts’ value of 7.3. Moreover, the continuous production of alkenols at industrial-related current density (−200 mA cm–2) over the efficient and durable Cu3Pd-based electrolyzer could achieve a cathodic energy efficiency of 45 mol kW·h–1, 1.7 times the bench-marked reactors, promising great potential for sustainable industrial synthesis.
中文翻译:

Cu3Pd 合金异质结中 Cuδ− 和 Pdδ+ 的电子发散促进了协调的 C≡C 键结合和炔醇半氢化的 Volmer 步骤
炔醇的电催化半氢化为烯醇的高价值生产提供了传统热方法的可持续替代方案。设计具有优异催化效率和能量效率的半加氢高效催化剂提出了重大挑战。在这里,我们提出了电子发散的Cu 3 Pd合金基异质结在促进以水作为质子源的炔醇电催化半氢化为烯醇中的应用。 Cu δ−和 Pd δ+的可调节电子散度,通过与富氮碳的整流接触进行调节,使得水解离的 Volmer 步骤中的活性 H 物种与 Pd δ+上的炔醇的 C≡C 键协同结合网站。同时,Cu 3 Pd 的显着电子发散促进了 Volmer 步骤中的 OH 物质和 Cu δ−位点上的炔醇的普遍吸附。电子发散双中心显着促进水解离并抑制完成氢的析出,使周转频率达到 2412 h –1 ,优于报道的电催化剂的值 7.3。此外,在高效耐用的Cu 3 Pd基电解槽上以工业相关电流密度(−200 mA cm –2 )连续生产烯醇,可实现45 mol kW·h –1的阴极能源效率,是现有技术的1.7倍。基准反应器,有望实现可持续工业合成的巨大潜力。
更新日期:2024-06-27
中文翻译:

Cu3Pd 合金异质结中 Cuδ− 和 Pdδ+ 的电子发散促进了协调的 C≡C 键结合和炔醇半氢化的 Volmer 步骤
炔醇的电催化半氢化为烯醇的高价值生产提供了传统热方法的可持续替代方案。设计具有优异催化效率和能量效率的半加氢高效催化剂提出了重大挑战。在这里,我们提出了电子发散的Cu 3 Pd合金基异质结在促进以水作为质子源的炔醇电催化半氢化为烯醇中的应用。 Cu δ−和 Pd δ+的可调节电子散度,通过与富氮碳的整流接触进行调节,使得水解离的 Volmer 步骤中的活性 H 物种与 Pd δ+上的炔醇的 C≡C 键协同结合网站。同时,Cu 3 Pd 的显着电子发散促进了 Volmer 步骤中的 OH 物质和 Cu δ−位点上的炔醇的普遍吸附。电子发散双中心显着促进水解离并抑制完成氢的析出,使周转频率达到 2412 h –1 ,优于报道的电催化剂的值 7.3。此外,在高效耐用的Cu 3 Pd基电解槽上以工业相关电流密度(−200 mA cm –2 )连续生产烯醇,可实现45 mol kW·h –1的阴极能源效率,是现有技术的1.7倍。基准反应器,有望实现可持续工业合成的巨大潜力。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号