当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Azabicyclo[3.1.1]heptenes Enabled by Catalyst-Controlled Annulations of Bicyclo[1.1.0]butanes with Vinyl Azides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-27 , DOI: 10.1021/jacs.4c04485 Zhongren Lin 1 , Haosong Ren 1 , Xinbo Lin 1 , Xinhong Yu 1 , Jun Zheng 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-27 , DOI: 10.1021/jacs.4c04485 Zhongren Lin 1 , Haosong Ren 1 , Xinbo Lin 1 , Xinhong Yu 1 , Jun Zheng 1
Affiliation
|
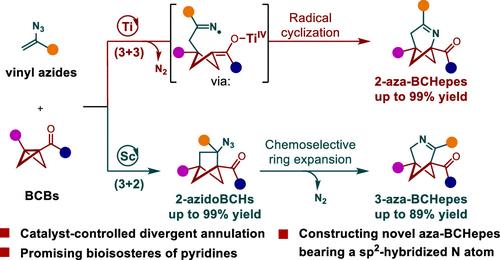
Bridged bicyclic scaffolds are emerging bioisosteres of planar aromatic rings under the concept of “escape from flatland”. However, adopting this concept into the exploration of bioisosteres of pyridines remains elusive due to the challenge of incorporating a N atom into such bridged bicyclic structures. Herein, we report practical routes for the divergent synthesis of 2- and 3-azabicyclo[3.1.1]heptenes (aza-BCHepes) as potential bioisosteres of pyridines from the readily accessible vinyl azides and bicyclo[1.1.0]butanes (BCBs) via two distinct catalytic annulations. The reactivity of vinyl azides tailored with BCBs is the key to achieving divergent transformations. TiIII-catalyzed single-electron reductive generation of C-radicals from BCBs allows a concise (3 + 3) annulation with vinyl azides, affording novel 2-aza-BCHepe scaffolds. In contrast, scandium catalysis enables an efficient dipolar (3 + 2) annulation with vinyl azides to generate 2-azidobicyclo[2.1.1]hexanes, which subsequently undergo a chemoselective rearrangement to construct 3-aza-BCHepes. Both approaches efficiently deliver unique azabicyclo[3.1.1]heptene scaffolds with a high functional group tolerance. The synthetic utility has been further demonstrated by scale-up reactions and diverse postcatalytic transformations, providing valuable azabicyclics including 2- and 3-azabicyclo[3.1.1]heptanes and rigid bicyclic amino esters. In addition, the related sp2-hybridized nitrogen atom and the similar geometric property between pyridines and corresponding aza-BCHepes indicate that they are promising bioisosteres of pyridines.
中文翻译:

通过催化剂控制双环[1.1.0]丁烷与乙烯基叠氮化物的环化合成氮杂双环[3.1.1]庚烯
桥联双环支架是在“逃离平地”概念下新兴的平面芳环生物等排体。然而,由于将氮原子纳入这种桥联双环结构的挑战,将这一概念应用于吡啶生物等排体的探索仍然难以实现。在此,我们报告了从容易获得的乙烯基叠氮化物和双环[1.1.0]丁烷(BCB)中不同合成2-和3-氮杂双环[3.1.1]庚烯(aza-BCHepes)作为吡啶潜在生物等排体的实用路线通过两个不同的催化环化。乙烯基叠氮化物与 BCB 的反应活性是实现不同转化的关键。 Ti III催化 BCB 的单电子还原生成 C 自由基,可以与乙烯基叠氮化物进行简洁的 (3 + 3) 成环,从而提供新型 2-aza-BCHepe 支架。相比之下,钪催化能够与乙烯基叠氮化物进行有效的偶极(3 + 2)环化,生成2-叠氮双环[2.1.1]己烷,随后进行化学选择性重排以构建3-氮杂-BCHepes。两种方法都有效地提供了具有高官能团耐受性的独特的氮杂双环[3.1.1]庚烯支架。通过放大反应和各种后催化转化进一步证明了合成实用性,提供了有价值的氮杂双环化合物,包括2-和3-氮杂双环[3.1.1]庚烷和刚性双环氨基酯。此外,吡啶和相应的aza-BCHepes之间相关的sp 2杂化氮原子和相似的几何性质表明它们是有前途的吡啶生物等排体。
更新日期:2024-06-27
中文翻译:

通过催化剂控制双环[1.1.0]丁烷与乙烯基叠氮化物的环化合成氮杂双环[3.1.1]庚烯
桥联双环支架是在“逃离平地”概念下新兴的平面芳环生物等排体。然而,由于将氮原子纳入这种桥联双环结构的挑战,将这一概念应用于吡啶生物等排体的探索仍然难以实现。在此,我们报告了从容易获得的乙烯基叠氮化物和双环[1.1.0]丁烷(BCB)中不同合成2-和3-氮杂双环[3.1.1]庚烯(aza-BCHepes)作为吡啶潜在生物等排体的实用路线通过两个不同的催化环化。乙烯基叠氮化物与 BCB 的反应活性是实现不同转化的关键。 Ti III催化 BCB 的单电子还原生成 C 自由基,可以与乙烯基叠氮化物进行简洁的 (3 + 3) 成环,从而提供新型 2-aza-BCHepe 支架。相比之下,钪催化能够与乙烯基叠氮化物进行有效的偶极(3 + 2)环化,生成2-叠氮双环[2.1.1]己烷,随后进行化学选择性重排以构建3-氮杂-BCHepes。两种方法都有效地提供了具有高官能团耐受性的独特的氮杂双环[3.1.1]庚烯支架。通过放大反应和各种后催化转化进一步证明了合成实用性,提供了有价值的氮杂双环化合物,包括2-和3-氮杂双环[3.1.1]庚烷和刚性双环氨基酯。此外,吡啶和相应的aza-BCHepes之间相关的sp 2杂化氮原子和相似的几何性质表明它们是有前途的吡啶生物等排体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号