当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transpersulfidation or H2S Release? Understanding the Landscape of Persulfide Chemical Biology
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-27 , DOI: 10.1021/jacs.4c05874 Kaylin G Fosnacht 1 , Jyoti Sharma 2 , Pier Alexandre Champagne 2 , Michael D Pluth 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-27 , DOI: 10.1021/jacs.4c05874 Kaylin G Fosnacht 1 , Jyoti Sharma 2 , Pier Alexandre Champagne 2 , Michael D Pluth 1
Affiliation
|
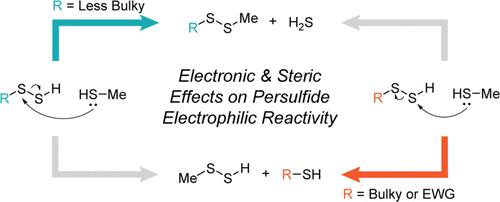
Persulfides (RSSH) are biologically important reactive sulfur species that are endogenously produced, protect key cysteine residues from irreversible oxidation, and are important intermediates during different enzymatic processes. Although persulfides are stronger nucleophiles than their thiol counterparts, persulfides can also act as electrophiles in their neutral, protonated form in specific environments. Moreover, persulfides are electrophilic at both sulfur atoms, and the reaction with a thiolate can lead to either H2S release with disulfide formation or alternatively result in transpersulfidation. Despite the broad acceptance of these reaction pathways, the specific properties that control whether persulfides react through the H2S-releasing or transpersulfidation pathway remain elusive. Herein, we use a combined computational and experimental approach to directly investigate the reactivity between persulfides and thiols to answer these questions. Using density functional theory (DFT) calculations, we demonstrate that increasing steric bulk or electron withdrawal near the persulfide can shunt persulfide reactivity through the transpersulfidation pathway. Building from these insights, we use a synthetic persulfide donor and an N-iodoacetyl l-tyrosine methyl ester (TME-IAM) trapping agent to experimentally monitor and measure transpersulfidation from a bulky penicillamine-based persulfide to a cysteine-based thiol, which, to the best of our knowledge, is the first direct observation of transpersulfidation between low-molecular-weight species. Taken together, these combined approaches highlight how the properties of persulfides are directly impacted by local environments, which has significant impacts in understanding the complex chemical biology of these reactive species.
中文翻译:

过硫化或 H2S 释放?了解过硫化物化学生物学的概况
过硫化物 (RSSH) 是生物学上重要的活性硫物质,是内源产生的,可保护关键半胱氨酸残基免受不可逆氧化,并且是不同酶促过程中的重要中间体。尽管过硫化物是比硫醇对应物更强的亲核试剂,但过硫化物在特定环境中也可以以其中性、质子化形式充当亲电子试剂。此外,过硫化物在两个硫原子上都是亲电子的,并且与硫醇盐的反应可以导致H 2 S释放并形成二硫化物,或者导致转过硫化。尽管这些反应途径已被广泛接受,但控制过硫化物是否通过 H 2 S 释放或转过硫化途径进行反应的具体性质仍然难以捉摸。在这里,我们使用计算和实验相结合的方法来直接研究过硫化物和硫醇之间的反应性来回答这些问题。使用密度泛函理论(DFT)计算,我们证明增加过硫化物附近的空间体积或吸电子可以通过转过硫化途径分流过硫化物反应性。基于这些见解,我们使用合成的过硫化物供体和N-碘乙酰基L-酪氨酸甲酯 (TME-IAM) 捕获剂来实验监测和测量从大体积青霉胺基过硫化物到半胱氨酸基硫醇的转过硫化作用,其中,据我们所知,这是首次直接观察到低分子量物质之间的过硫化作用。 总而言之,这些组合方法强调了过硫化物的性质如何直接受到当地环境的影响,这对于理解这些活性物质的复杂化学生物学具有重大影响。
更新日期:2024-06-27
中文翻译:

过硫化或 H2S 释放?了解过硫化物化学生物学的概况
过硫化物 (RSSH) 是生物学上重要的活性硫物质,是内源产生的,可保护关键半胱氨酸残基免受不可逆氧化,并且是不同酶促过程中的重要中间体。尽管过硫化物是比硫醇对应物更强的亲核试剂,但过硫化物在特定环境中也可以以其中性、质子化形式充当亲电子试剂。此外,过硫化物在两个硫原子上都是亲电子的,并且与硫醇盐的反应可以导致H 2 S释放并形成二硫化物,或者导致转过硫化。尽管这些反应途径已被广泛接受,但控制过硫化物是否通过 H 2 S 释放或转过硫化途径进行反应的具体性质仍然难以捉摸。在这里,我们使用计算和实验相结合的方法来直接研究过硫化物和硫醇之间的反应性来回答这些问题。使用密度泛函理论(DFT)计算,我们证明增加过硫化物附近的空间体积或吸电子可以通过转过硫化途径分流过硫化物反应性。基于这些见解,我们使用合成的过硫化物供体和N-碘乙酰基L-酪氨酸甲酯 (TME-IAM) 捕获剂来实验监测和测量从大体积青霉胺基过硫化物到半胱氨酸基硫醇的转过硫化作用,其中,据我们所知,这是首次直接观察到低分子量物质之间的过硫化作用。 总而言之,这些组合方法强调了过硫化物的性质如何直接受到当地环境的影响,这对于理解这些活性物质的复杂化学生物学具有重大影响。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号