当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Situ Forming, Enzyme-Responsive Peptoid-Peptide Hydrogels: An Advanced Long-Acting Injectable Drug Delivery System
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-26 , DOI: 10.1021/jacs.4c03751 Sophie M. Coulter 1 , Sreekanth Pentlavalli 1 , Yuming An 1 , Lalitkumar K. Vora 1 , Emily R. Cross 1 , Jessica V. Moore 1 , Han Sun 1 , Ralf Schweins 2 , Helen O. McCarthy 1 , Garry Laverty 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-26 , DOI: 10.1021/jacs.4c03751 Sophie M. Coulter 1 , Sreekanth Pentlavalli 1 , Yuming An 1 , Lalitkumar K. Vora 1 , Emily R. Cross 1 , Jessica V. Moore 1 , Han Sun 1 , Ralf Schweins 2 , Helen O. McCarthy 1 , Garry Laverty 1
Affiliation
|
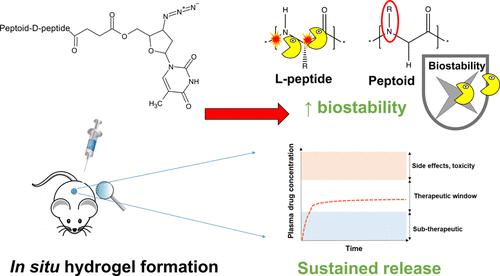
Long-acting drug delivery systems are promising platforms to improve patient adherence to medication by delivering drugs over sustained periods and removing the need for patients to comply with oral regimens. This research paper provides a proof-of-concept for the development of a new optimized in situ forming injectable depot based on a tetrabenzylamine-tetraglycine-d-lysine-O-phospho-d-tyrosine peptoid-D-peptide formulation ((NPhe)4GGGGk(AZT)y(p)-OH). The chemical versatility of the peptoid-peptide motif allows low-molecular-weight drugs to be precisely and covalently conjugated. After subcutaneous injection, a hydrogel depot forms from the solubilized peptoid-peptide-drug formulation in response to phosphatase enzymes present within the skin space. This system is able to deliver clinically relevant concentrations of a model drug, the antiretroviral zidovudine (AZT), for 35 days in Sprague–Dawley rats. Oscillatory rheology demonstrated that hydrogel formation began within ∼30 s, an important characteristic of in situ systems for reducing initial drug bursts. Gel formation continued for up to ∼90 min. Small-angle neutron scattering data reveal narrow-radius fibers (∼0.78–1.8 nm) that closely fit formation via a flexible cylinder elliptical model. The inclusion of non-native peptoid monomers and D-variant amino acids confers protease resistance, enabling enhanced biostability to be demonstrated in vitro. Drug release proceeds via hydrolysis of an ester linkage under physiological conditions, releasing the drug in an unmodified form and further reducing the initial drug burst. Subcutaneous administration of (NPhe)4GGGGk(AZT)y(p)-OH to Sprague–Dawley rats resulted in zidovudine blood plasma concentrations within the 90% maximal inhibitory concentration (IC90) range (30–130 ng mL–1) for 35 days.
中文翻译:

原位形成酶响应肽-肽水凝胶:一种先进的长效注射给药系统
长效药物输送系统是一种很有前途的平台,可以通过持续输送药物并消除患者遵守口服方案的需要来提高患者对药物的依从性。本研究论文为开发基于四苄胺-四甘氨酸-d-赖氨酸-O-磷酸-d-酪氨酸肽-D-肽制剂((NPhe) 4 GGGGk(AZT)y(p)-OH)。类肽-肽基序的化学多功能性使得低分子量药物能够精确地共价结合。皮下注射后,响应皮肤空间内存在的磷酸酶,由溶解的类肽-肽-药物制剂形成水凝胶储库。该系统能够在 Sprague-Dawley 大鼠体内提供临床相关浓度的模型药物抗逆转录病毒齐多夫定 (AZT),持续 35 天。振荡流变学表明,水凝胶在~30秒内开始形成,这是减少初始药物爆发的原位系统的一个重要特征。凝胶形成持续约 90 分钟。小角度中子散射数据揭示了窄半径纤维(∼0.78–1.8 nm),通过灵活的圆柱椭圆模型紧密贴合地层。包含非天然类肽单体和 D 变体氨基酸赋予蛋白酶抗性,从而能够在体外证明增强的生物稳定性。药物释放通过生理条件下酯键的水解进行,以未修饰的形式释放药物并进一步减少初始药物突释。 对 Sprague–Dawley 大鼠皮下注射 (NPhe) 4 GGGGk(AZT)y(p)-OH 导致齐多夫定血浆浓度在 90% 最大抑制浓度内 (IC 90 ) 范围 (30–130 ng mL –1 ) 35 天。
更新日期:2024-06-26
中文翻译:

原位形成酶响应肽-肽水凝胶:一种先进的长效注射给药系统
长效药物输送系统是一种很有前途的平台,可以通过持续输送药物并消除患者遵守口服方案的需要来提高患者对药物的依从性。本研究论文为开发基于四苄胺-四甘氨酸-d-赖氨酸-O-磷酸-d-酪氨酸肽-D-肽制剂((NPhe) 4 GGGGk(AZT)y(p)-OH)。类肽-肽基序的化学多功能性使得低分子量药物能够精确地共价结合。皮下注射后,响应皮肤空间内存在的磷酸酶,由溶解的类肽-肽-药物制剂形成水凝胶储库。该系统能够在 Sprague-Dawley 大鼠体内提供临床相关浓度的模型药物抗逆转录病毒齐多夫定 (AZT),持续 35 天。振荡流变学表明,水凝胶在~30秒内开始形成,这是减少初始药物爆发的原位系统的一个重要特征。凝胶形成持续约 90 分钟。小角度中子散射数据揭示了窄半径纤维(∼0.78–1.8 nm),通过灵活的圆柱椭圆模型紧密贴合地层。包含非天然类肽单体和 D 变体氨基酸赋予蛋白酶抗性,从而能够在体外证明增强的生物稳定性。药物释放通过生理条件下酯键的水解进行,以未修饰的形式释放药物并进一步减少初始药物突释。 对 Sprague–Dawley 大鼠皮下注射 (NPhe) 4 GGGGk(AZT)y(p)-OH 导致齐多夫定血浆浓度在 90% 最大抑制浓度内 (IC 90 ) 范围 (30–130 ng mL –1 ) 35 天。











































 京公网安备 11010802027423号
京公网安备 11010802027423号