当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Formation of Metathesis-Active Tungsten Alkylidene Complexes from Cyclohexene
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-25 , DOI: 10.1021/jacs.4c05256 Milan Maji 1 , Adenilson Sousa-Silva 2 , Xavier Solans-Monfort 2 , Richard R Schrock 1 , Matthew P Conley 1 , Phillip Farias 1 , Veronica Carta 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-25 , DOI: 10.1021/jacs.4c05256 Milan Maji 1 , Adenilson Sousa-Silva 2 , Xavier Solans-Monfort 2 , Richard R Schrock 1 , Matthew P Conley 1 , Phillip Farias 1 , Veronica Carta 1
Affiliation
|
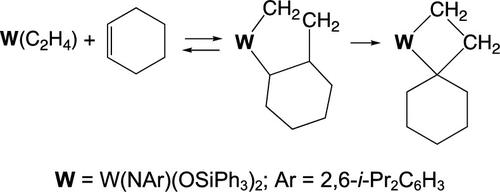
A 7-tungstabicyclo[4.3.0]nonane complex forms slowly upon addition of cyclohexene to the ethylene complex, W(NAr)(OSiPh3)2(C2H4), at 22 °C. A single-crystal X-ray study showed its structure to be closest to a square pyramid (τ = 0.23). At 22 °C, loss of cyclohexene or ring contraction of the 7-tungstabicyclo[4.3.0]nonane complex is slow. Above ∼80 °C, cyclohexene is ejected to give W(NAr)(OSiPh3)2(C2H4), but a sufficient amount of 7-tungstabicyclo[4.3.0]nonane complex remains in the presence of cyclohexene and the ring contracts to yield methylenecyclohexane and a methylidene complex or ethylene and a cyclohexylidene complex. Other complexes that have been observed include an 8-tungstabicyclo[4.3.0]nonane complex formed from 1,7-octadiene, a 7-tungstabicyclo[4.2.0]octane complex (formed from a methylidene complex and cyclohexene), and a methylenecyclohexane complex. 13C-Labeling studies show that the exo-methylene group in methylenecyclohexane and the α positions in the 8-tungstabicyclo[4.3.0]nonane come from ethylene. An alternative ring contraction of a tungstacyclopentane made from two molecules of cyclohexene cannot be excluded when concentrations of ethylene are low. A cyclohexylidene complex could also form from two cyclohexenes via a newly proposed “alkyl/allyl” mechanism. The results reported here are the first experimental confirmations that a tungstacyclopentane can ring-contract thermally at a substituted WCα position to form a tungstacyclobutane and therefore metathesis-active alkylidenes.
中文翻译:

环己烯热形成复分解活性亚烷基钨配合物
在 22 °C 下,将环己烯添加到乙烯络合物 W(NAr)(OSiPh 3 ) 2 (C 2 H 4 ) 中后,缓慢形成 7-钨双环[4.3.0]壬烷络合物。单晶 X 射线研究表明其结构最接近四角锥体 (τ = 0.23)。在 22 °C 时,环己烯的损失或 7-钨双环[4.3.0]壬烷络合物的环收缩很慢。在~80℃以上,环己烯被喷出,得到W(NAr)(OSiPh 3 ) 2 (C 2 H 4 ),但在环己烯存在下仍保留足够量的7-钨双环[4.3.0]壬烷络合物环收缩产生亚甲基环己烷和亚甲基络合物或乙烯和亚环己基络合物。已观察到的其他配合物包括由1,7-辛二烯形成的8-钨双环[4.3.0]壬烷配合物、7-钨双环[4.2.0]辛烷配合物(由亚甲基配合物和环己烯形成)和亚甲基环己烷复杂的。 13 C 标记研究表明,亚甲基环己烷中的外亚甲基和 8-钨双环[4.3.0]壬烷中的 α 位来自乙烯。当乙烯浓度较低时,不能排除由两个环己烯分子制成的钨环戊烷的替代环收缩。亚环己基络合物也可以通过新提出的“烷基/烯丙基”机制由两个环己烯形成。这里报告的结果是首次实验证实钨环戊烷可以在取代的 WC α位热收缩形成钨环丁烷,从而形成复分解活性亚烷基。
更新日期:2024-06-25
中文翻译:

环己烯热形成复分解活性亚烷基钨配合物
在 22 °C 下,将环己烯添加到乙烯络合物 W(NAr)(OSiPh 3 ) 2 (C 2 H 4 ) 中后,缓慢形成 7-钨双环[4.3.0]壬烷络合物。单晶 X 射线研究表明其结构最接近四角锥体 (τ = 0.23)。在 22 °C 时,环己烯的损失或 7-钨双环[4.3.0]壬烷络合物的环收缩很慢。在~80℃以上,环己烯被喷出,得到W(NAr)(OSiPh 3 ) 2 (C 2 H 4 ),但在环己烯存在下仍保留足够量的7-钨双环[4.3.0]壬烷络合物环收缩产生亚甲基环己烷和亚甲基络合物或乙烯和亚环己基络合物。已观察到的其他配合物包括由1,7-辛二烯形成的8-钨双环[4.3.0]壬烷配合物、7-钨双环[4.2.0]辛烷配合物(由亚甲基配合物和环己烯形成)和亚甲基环己烷复杂的。 13 C 标记研究表明,亚甲基环己烷中的外亚甲基和 8-钨双环[4.3.0]壬烷中的 α 位来自乙烯。当乙烯浓度较低时,不能排除由两个环己烯分子制成的钨环戊烷的替代环收缩。亚环己基络合物也可以通过新提出的“烷基/烯丙基”机制由两个环己烯形成。这里报告的结果是首次实验证实钨环戊烷可以在取代的 WC α位热收缩形成钨环丁烷,从而形成复分解活性亚烷基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号