当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mining the Carbon Intermediates in Plastic Waste Upcycling for Constructing C–S Bond
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-25 , DOI: 10.1021/jacs.4c05512 Hongxing Kang 1 , Dong He 2 , Christopher Turchiano 1 , Xingxu Yan 3 , Jingtong Chai 1 , Melanie Weed 1 , Gregory I Elliott 1 , David Onofrei 1 , Xiaoqing Pan 3, 4 , Xiangheng Xiao 2 , Jing Gu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-25 , DOI: 10.1021/jacs.4c05512 Hongxing Kang 1 , Dong He 2 , Christopher Turchiano 1 , Xingxu Yan 3 , Jingtong Chai 1 , Melanie Weed 1 , Gregory I Elliott 1 , David Onofrei 1 , Xiaoqing Pan 3, 4 , Xiangheng Xiao 2 , Jing Gu 1
Affiliation
|
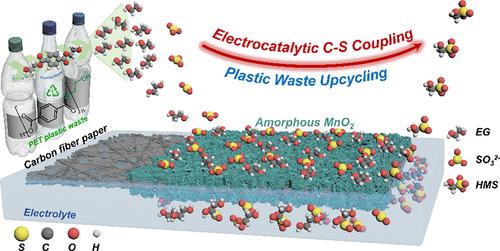
Postconsumer plastics are generally perceived as valueless with only a small portion of plastic waste being closed-loop recycled into similar products while most of them are discarded in landfills. Depositing plastic waste in landfills not only harms the environment but also signifies a substantial economic loss. Alternatively, constructing value-added chemical feedstocks via mining the waste-derived intermediate species as a carbon (C) source under mild electrochemical conditions is a sustainable strategy to realize the circular economy. This proof-of-concept work provides an attractive “turning trash to treasure” strategy by integrating electrocatalytic polyethylene terephthalate (PET) plastic upcycling with a chemical C–S coupling reaction to synthesize organosulfur compounds, hydroxymethanesulfonate (HMS). HMS can be produced efficiently (Faradaic efficiency, FE of ∼70%) via deliberately capturing electrophilic intermediates generated in the PET monomer (ethylene glycol, EG) upcycling process, followed by coupling them with nucleophilic sulfur (S) species (i.e., SO32– and HSO3–). Unlike many previous studies conducted under alkaline conditions, PET upcycling was performed over an amorphous MnO2 catalyst under near-neutral conditions, allowing for the stabilization of electrophilic intermediates. The compatibility of this strategy was further investigated by employing biomass-derived compounds as substrates. Moreover, comparable HMS yields can be achieved with real-world PET plastics, showing its enormous potential in practical application. Lastly, Density function theory (DFT) calculation reveals that the C–C cleavage step of EG is the rate-determining step (RDS), and amorphous MnO2 significantly decreases the energy barriers for both RDS and C–S coupling when compared to the crystalline counterpart.
中文翻译:

挖掘塑料垃圾升级回收中的碳中间体构建C-S键
消费后塑料通常被认为毫无价值,只有一小部分塑料废物被闭环回收成类似产品,而大多数则被丢弃在垃圾填埋场。将塑料废物存放在垃圾填埋场不仅危害环境,而且还意味着巨大的经济损失。或者,通过在温和的电化学条件下开采废物衍生的中间物质作为碳(C)源来构建增值化学原料是实现循环经济的可持续策略。这项概念验证工作通过将电催化聚对苯二甲酸乙二醇酯 (PET) 塑料升级回收与化学 C-S 偶联反应相结合来合成有机硫化合物羟基甲磺酸 (HMS),提供了一种有吸引力的“变废为宝”策略。通过故意捕获 PET 单体(乙二醇,EG)升级过程中产生的亲电子中间体,然后将它们与亲核硫 (S) 物质(即 SO 3 2–和 HSO 3 – )。与之前在碱性条件下进行的许多研究不同,PET升级回收是在接近中性条件下在无定形MnO 2催化剂上进行的,从而实现了亲电中间体的稳定。通过使用生物质衍生的化合物作为底物,进一步研究了该策略的兼容性。此外,现实世界中的 PET 塑料也能实现与 HMS 相当的产量,显示出其在实际应用中的巨大潜力。 最后,密度函数理论(DFT)计算表明,EG的C-C裂解步骤是速率决定步骤(RDS),与非晶态MnO 2 相比,非晶态MnO 2显着降低了RDS和C-S耦合的能垒。结晶对应物。
更新日期:2024-06-25
中文翻译:

挖掘塑料垃圾升级回收中的碳中间体构建C-S键
消费后塑料通常被认为毫无价值,只有一小部分塑料废物被闭环回收成类似产品,而大多数则被丢弃在垃圾填埋场。将塑料废物存放在垃圾填埋场不仅危害环境,而且还意味着巨大的经济损失。或者,通过在温和的电化学条件下开采废物衍生的中间物质作为碳(C)源来构建增值化学原料是实现循环经济的可持续策略。这项概念验证工作通过将电催化聚对苯二甲酸乙二醇酯 (PET) 塑料升级回收与化学 C-S 偶联反应相结合来合成有机硫化合物羟基甲磺酸 (HMS),提供了一种有吸引力的“变废为宝”策略。通过故意捕获 PET 单体(乙二醇,EG)升级过程中产生的亲电子中间体,然后将它们与亲核硫 (S) 物质(即 SO 3 2–和 HSO 3 – )。与之前在碱性条件下进行的许多研究不同,PET升级回收是在接近中性条件下在无定形MnO 2催化剂上进行的,从而实现了亲电中间体的稳定。通过使用生物质衍生的化合物作为底物,进一步研究了该策略的兼容性。此外,现实世界中的 PET 塑料也能实现与 HMS 相当的产量,显示出其在实际应用中的巨大潜力。 最后,密度函数理论(DFT)计算表明,EG的C-C裂解步骤是速率决定步骤(RDS),与非晶态MnO 2 相比,非晶态MnO 2显着降低了RDS和C-S耦合的能垒。结晶对应物。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号