当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical Reduction of N2 to Ammonia Promoted by Hydrated Cation Ions: Mechanistic Insights from a Combined Computational and Experimental Study
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-25 , DOI: 10.1021/jacs.4c06629 Xin Mao 1, 2 , Xiaowan Bai 2 , Guanzheng Wu 3 , Qing Qin 3 , Anthony P. O’Mullane 1 , Yan Jiao 2 , Aijun Du 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-25 , DOI: 10.1021/jacs.4c06629 Xin Mao 1, 2 , Xiaowan Bai 2 , Guanzheng Wu 3 , Qing Qin 3 , Anthony P. O’Mullane 1 , Yan Jiao 2 , Aijun Du 1
Affiliation
|
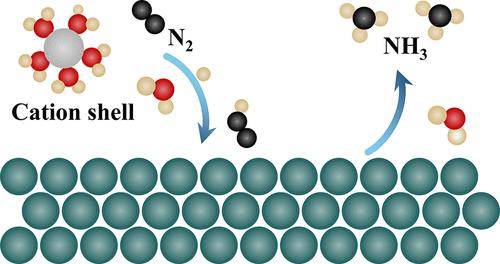
Alkali ions, major components at the electrode–electrolyte interface, are crucial to modulating reaction activity and selectivity of catalyst materials. However, the underlying mechanism of how the alkali ions catalyze the N2 reduction reaction (NRR) into ammonia remains elusive, posing challenges for experimentalists to select appropriate electrolyte solutions. In this work, by employing a combined experimental and computational approach, we proposed four essential roles of cation ions at Fe electrodes for N2 fixation: (i) promoting NN bond cleavage; (ii) stabilizing NRR intermediates; (iii) suppressing the competing hydrogen evolution reaction (HER); and (iv) modulating the interfacial charge distribution at the electrode–electrolyte interface. For N2 adsorption on an Fe electrode with cation ions, our constrained ab initio molecular dynamic (c-AIMD) results demonstrate a barrierless process, while an extra 0.52 eV barrier requires to be overcome to adsorb N2 for the pure Fe-water interface. For the formation of *NNH species within the N2 reduction process, the calculated free energy barrier is 0.50 eV at the Li+-Fe-water interface. However, the calculated barrier reaches 0.81 eV in pure Fe-water interface. Furthermore, experiments demonstrate a high Faradaic efficiency for ammonia synthesis on a Li+-Fe-water interface, reaching 27.93% at a working potential of −0.3 V vs RHE and pH = 6.8. These results emphasize how alkali metal cations and local reaction environments on the electrode surface play crucial roles in influencing the kinetics of interfacial reactions.
中文翻译:

水合阳离子促进 N2 电化学还原为氨:计算和实验相结合的研究的机理见解
碱离子是电极-电解质界面的主要成分,对于调节催化剂材料的反应活性和选择性至关重要。然而,碱离子如何催化 N 2 还原反应(NRR)生成氨的基本机制仍然难以捉摸,这给实验人员选择合适的电解质溶液带来了挑战。在这项工作中,通过采用实验和计算相结合的方法,我们提出了 Fe 电极上的阳离子对 N 2 固定的四个重要作用:(i)促进 NN 键断裂; (ii) 稳定NRR中间体; (iii) 抑制竞争性析氢反应(HER); (iv) 调节电极-电解质界面处的界面电荷分布。对于带有阳离子的 Fe 电极上的 N 2 吸附,我们的约束从头算分子动力学 (c-AIMD) 结果证明了无势垒过程,而需要克服额外的 0.52 eV 势垒才能吸附 N 2 为纯铁-水界面。对于 N 2 还原过程中 *NNH 物种的形成,计算得出 Li + -Fe-水界面处的自由能垒为 0.50 eV。然而,计算得出的纯铁-水界面势垒达到0.81 eV。此外,实验表明,Li + -Fe-水界面上的氨合成具有较高的法拉第效率,在 -0.3 V vs RHE 和 pH = 6.8 的工作电位下达到 27.93%。这些结果强调了碱金属阳离子和电极表面的局部反应环境在影响界面反应动力学方面发挥着至关重要的作用。
更新日期:2024-06-25
中文翻译:

水合阳离子促进 N2 电化学还原为氨:计算和实验相结合的研究的机理见解
碱离子是电极-电解质界面的主要成分,对于调节催化剂材料的反应活性和选择性至关重要。然而,碱离子如何催化 N 2 还原反应(NRR)生成氨的基本机制仍然难以捉摸,这给实验人员选择合适的电解质溶液带来了挑战。在这项工作中,通过采用实验和计算相结合的方法,我们提出了 Fe 电极上的阳离子对 N 2 固定的四个重要作用:(i)促进 NN 键断裂; (ii) 稳定NRR中间体; (iii) 抑制竞争性析氢反应(HER); (iv) 调节电极-电解质界面处的界面电荷分布。对于带有阳离子的 Fe 电极上的 N 2 吸附,我们的约束从头算分子动力学 (c-AIMD) 结果证明了无势垒过程,而需要克服额外的 0.52 eV 势垒才能吸附 N 2 为纯铁-水界面。对于 N 2 还原过程中 *NNH 物种的形成,计算得出 Li + -Fe-水界面处的自由能垒为 0.50 eV。然而,计算得出的纯铁-水界面势垒达到0.81 eV。此外,实验表明,Li + -Fe-水界面上的氨合成具有较高的法拉第效率,在 -0.3 V vs RHE 和 pH = 6.8 的工作电位下达到 27.93%。这些结果强调了碱金属阳离子和电极表面的局部反应环境在影响界面反应动力学方面发挥着至关重要的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号