当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enzyme-like Acyl Transfer Catalysis in a Bifunctional Organic Cage
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-24 , DOI: 10.1021/jacs.4c03560 Keith G. Andrews 1, 2 , Tomasz K. Piskorz 1 , Peter N. Horton 3 , Simon J. Coles 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-24 , DOI: 10.1021/jacs.4c03560 Keith G. Andrews 1, 2 , Tomasz K. Piskorz 1 , Peter N. Horton 3 , Simon J. Coles 3
Affiliation
|
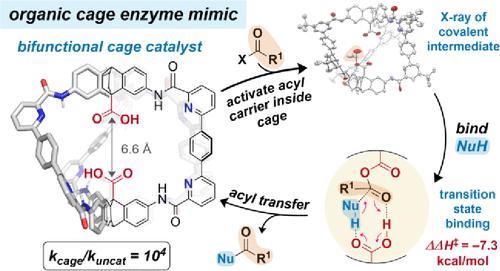
Amide-based organic cage cavities are, in principle, ideal enzyme active site mimics. Yet, cage-promoted organocatalysis has remained elusive, in large part due to synthetic accessibility of robust and functional scaffolds. Herein, we report the acyl transfer catalysis properties of robust, hexaamide cages in organic solvent. Cage structural variation reveals that esterification catalysis with an acyl anhydride acyl carrier occurs only in bifunctional cages featuring internal pyridine motifs and two crucial antipodal carboxylic acid groups. 1H NMR data and X-ray crystallography show that the acyl carrier is rapidly activated inside the cavity as a covalent mixed-anhydride intermediate with an internal hydrogen bond. Michaelis–Menten (saturation) kinetics suggest weak binding (KM = 0.16 M) of the alcohol pronucleophile close to the internal anhydride. Finally, activation and delivery of the alcohol to the internal anhydride by the second carboxylic acid group forms ester product and releases the cage catalyst. Eyring analysis indicates a strong enthalpic stabilization of the transition state (5.5 kcal/mol) corresponding to a rate acceleration of 104 over background acylation, and an ordered, associative rate-determining attack by the alcohol, supported by DFT calculations. We conclude that internal bifunctional organocatalysis specific to the cage structural design is responsible for the enhancement over the background reaction. These results pave the way for organic-phase enzyme mimicry in self-assembled cavities with the potential for cavity elaboration to enact selective acylations.
中文翻译:

双功能有机笼中的类酶酰基转移催化
原则上,基于酰胺的有机笼腔是理想的酶活性位点模拟物。然而,笼促进的有机催化仍然难以捉摸,这在很大程度上是由于坚固且功能性支架的合成可及性。在此,我们报告了有机溶剂中坚固的六酰胺笼的酰基转移催化性能。笼结构变化表明,与酰酐酰基载体的酯化催化仅发生在具有内部吡啶基序和两个关键反足羧酸基团的双功能笼中。 1 H NMR数据和X射线晶体学表明,酰基载体作为具有内部氢键的共价混合酸酐中间体在空腔内快速活化。 Michaelis-Menten(饱和)动力学表明靠近内酸酐的醇亲核试剂的结合较弱(K M = 0.16 M)。最后,通过第二个羧酸基团将醇活化并传递至内酸酐,形成酯产物并释放笼状催化剂。 Eyring 分析表明,过渡态 (5.5 kcal/mol) 具有很强的焓稳定性,对应于背景酰化作用下 10 4 的速率加速,以及由醇支持的有序、关联的速率决定攻击。 DFT 计算。我们得出的结论是,笼结构设计特有的内部双功能有机催化作用增强了背景反应。这些结果为自组装空腔中的有机相酶模拟铺平了道路,并有可能通过空腔精细化来实施选择性酰化。
更新日期:2024-06-24
中文翻译:

双功能有机笼中的类酶酰基转移催化
原则上,基于酰胺的有机笼腔是理想的酶活性位点模拟物。然而,笼促进的有机催化仍然难以捉摸,这在很大程度上是由于坚固且功能性支架的合成可及性。在此,我们报告了有机溶剂中坚固的六酰胺笼的酰基转移催化性能。笼结构变化表明,与酰酐酰基载体的酯化催化仅发生在具有内部吡啶基序和两个关键反足羧酸基团的双功能笼中。 1 H NMR数据和X射线晶体学表明,酰基载体作为具有内部氢键的共价混合酸酐中间体在空腔内快速活化。 Michaelis-Menten(饱和)动力学表明靠近内酸酐的醇亲核试剂的结合较弱(K M = 0.16 M)。最后,通过第二个羧酸基团将醇活化并传递至内酸酐,形成酯产物并释放笼状催化剂。 Eyring 分析表明,过渡态 (5.5 kcal/mol) 具有很强的焓稳定性,对应于背景酰化作用下 10 4 的速率加速,以及由醇支持的有序、关联的速率决定攻击。 DFT 计算。我们得出的结论是,笼结构设计特有的内部双功能有机催化作用增强了背景反应。这些结果为自组装空腔中的有机相酶模拟铺平了道路,并有可能通过空腔精细化来实施选择性酰化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号