当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
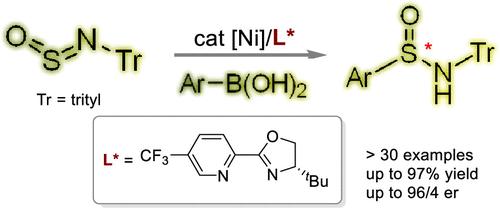
Asymmetric 2,3-Addition of Sulfinylamines with Arylboronic Acids Enabled by Nickel Catalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-24 , DOI: 10.1021/jacs.4c04050 Longlong Xi 1 , Xiaowu Fang 1 , Minyan Wang 1 , Zhuangzhi Shi 1, 2, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-24 , DOI: 10.1021/jacs.4c04050 Longlong Xi 1 , Xiaowu Fang 1 , Minyan Wang 1 , Zhuangzhi Shi 1, 2, 3
Affiliation
|
Sulfinamides have been widely used in organic synthesis, with research on their preparation spanning more than a century. Despite advancements in catalytic methodologies, creating sulfur stereocenters within these molecules remains a significant challenge. In this study, we present an effective and versatile method for synthesizing a diverse range of S-chirogenic sulfinamides through catalytic asymmetric aryl addition to sulfinylamines. By utilizing a nickel complex as a catalyst, this process exhibits impressive enantioselectivity and can incorporate various arylboronic acids at the sulfur position. The resulting synthetic sulfinamides are stable and highly adaptable, allowing for their conversion to a variety of sulfur-containing compounds. Our study also incorporates detailed experimental and computational studies to elucidate the reaction mechanism and factors influencing enantioselectivity.
中文翻译:

镍催化下亚磺胺与芳基硼酸的不对称 2,3-加成
亚磺酰胺已广泛应用于有机合成中,对其制备的研究已有一个多世纪的历史。尽管催化方法取得了进步,但在这些分子内创建硫立构中心仍然是一个重大挑战。在这项研究中,我们提出了一种有效且通用的方法,通过催化不对称芳基加成到亚磺胺上来合成各种 S-手性亚磺酰胺。通过利用镍络合物作为催化剂,该过程表现出令人印象深刻的对映选择性,并且可以在硫位置结合各种芳基硼酸。所得的合成亚磺酰胺稳定且适应性强,可以转化为多种含硫化合物。我们的研究还结合了详细的实验和计算研究,以阐明反应机理和影响对映选择性的因素。
更新日期:2024-06-24
中文翻译:

镍催化下亚磺胺与芳基硼酸的不对称 2,3-加成
亚磺酰胺已广泛应用于有机合成中,对其制备的研究已有一个多世纪的历史。尽管催化方法取得了进步,但在这些分子内创建硫立构中心仍然是一个重大挑战。在这项研究中,我们提出了一种有效且通用的方法,通过催化不对称芳基加成到亚磺胺上来合成各种 S-手性亚磺酰胺。通过利用镍络合物作为催化剂,该过程表现出令人印象深刻的对映选择性,并且可以在硫位置结合各种芳基硼酸。所得的合成亚磺酰胺稳定且适应性强,可以转化为多种含硫化合物。我们的研究还结合了详细的实验和计算研究,以阐明反应机理和影响对映选择性的因素。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号