当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineering a Robust UDP-Glucose Pyrophosphorylase for Enhanced Biocatalytic Synthesis via ProteinMPNN and Ancestral Sequence Reconstruction
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-06-25 , DOI: 10.1021/acs.jafc.4c03126 Zonglin Li 1 , Miaozi Lou 1 , Chuanqi Sun 1 , Zhimin Li 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-06-25 , DOI: 10.1021/acs.jafc.4c03126 Zonglin Li 1 , Miaozi Lou 1 , Chuanqi Sun 1 , Zhimin Li 1, 2
Affiliation
|
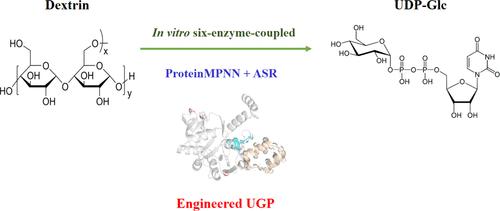
UDP-glucose is a key metabolite in carbohydrate metabolism and plays a vital role in glycosyl transfer reactions. Its significance spans across the food and agricultural industries. This study focuses on UDP-glucose synthesis via multienzyme catalysis using dextrin, incorporating UTP production and ATP regeneration modules to reduce costs. To address thermal stability limitations of the key UDP-glucose pyrophosphorylase (UGP), a deep learning-based protein sequence design approach and ancestral sequence reconstruction are employed to engineer a thermally stable UGP variant. The engineered UGP variant is significantly 500-fold more thermally stable at 60 °C and has a half-life of 49.8 h compared to the wild-type enzyme. MD simulations and umbrella sampling calculations provide insights into the mechanism behind the enhanced thermal stability. Experimental validation demonstrates that the engineered UGP variant can produce 52.6 mM UDP-glucose within 6 h in an in vitro cascade reaction. This study offers practical insights for efficient UDP-glucose synthesis methods.
中文翻译:

通过 ProteinMPNN 和祖先序列重建设计强大的 UDP-葡萄糖焦磷酸化酶以增强生物催化合成
UDP-葡萄糖是碳水化合物代谢中的关键代谢物,在糖基转移反应中起着至关重要的作用。它的重要性遍及食品和农业行业。本研究重点是使用糊精通过多酶催化合成 UDP-葡萄糖,结合 UTP 生产和 ATP 再生模块以降低成本。为了解决关键 UDP-葡萄糖焦磷酸化酶 (UGP) 的热稳定性限制,采用基于深度学习的蛋白质序列设计方法和祖先序列重建来设计热稳定的 UGP 变体。与野生型酶相比,工程 UGP 变体在 60°C 下的热稳定性显着提高了 500 倍,半衰期为 49.8 小时。 MD 模拟和伞式采样计算提供了对增强热稳定性背后机制的深入了解。实验验证表明,工程 UGP 变体可以在体外级联反应中在 6 小时内产生 52.6 mM UDP-葡萄糖。这项研究为有效的 UDP-葡萄糖合成方法提供了实用的见解。
更新日期:2024-06-25
中文翻译:

通过 ProteinMPNN 和祖先序列重建设计强大的 UDP-葡萄糖焦磷酸化酶以增强生物催化合成
UDP-葡萄糖是碳水化合物代谢中的关键代谢物,在糖基转移反应中起着至关重要的作用。它的重要性遍及食品和农业行业。本研究重点是使用糊精通过多酶催化合成 UDP-葡萄糖,结合 UTP 生产和 ATP 再生模块以降低成本。为了解决关键 UDP-葡萄糖焦磷酸化酶 (UGP) 的热稳定性限制,采用基于深度学习的蛋白质序列设计方法和祖先序列重建来设计热稳定的 UGP 变体。与野生型酶相比,工程 UGP 变体在 60°C 下的热稳定性显着提高了 500 倍,半衰期为 49.8 小时。 MD 模拟和伞式采样计算提供了对增强热稳定性背后机制的深入了解。实验验证表明,工程 UGP 变体可以在体外级联反应中在 6 小时内产生 52.6 mM UDP-葡萄糖。这项研究为有效的 UDP-葡萄糖合成方法提供了实用的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号