当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetics of Methyl Methacrylate Polymerization in the Presence of Initiating Systems “Peroxide + Zirconocene Dichloride” When the Methyl Methacrylate Adhesive is Cured
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-26 , DOI: 10.1021/acs.iecr.4c01937 Konstantin A. Tereshchenko 1 , Daria A. Shiyan 1 , Andrey A. Osipov 1 , Vera P. Bondarenko 1 , Nikolai V. Ulitin 1 , Elina M. Sabitova 1 , Anton V. Bekker 1 , Yana L. Lyulinskaya 1 , Nikolay A. Novikov 1 , Natalia M. Nurullina 1 , Svetlana N. Tuntseva 1 , Tatyana L. Puchkova 1 , Yaroslav O. Mezhuev 2, 3 , Kharlampii E. Kharlampidi 1 , Sergey V. Kolesov 4
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-26 , DOI: 10.1021/acs.iecr.4c01937 Konstantin A. Tereshchenko 1 , Daria A. Shiyan 1 , Andrey A. Osipov 1 , Vera P. Bondarenko 1 , Nikolai V. Ulitin 1 , Elina M. Sabitova 1 , Anton V. Bekker 1 , Yana L. Lyulinskaya 1 , Nikolay A. Novikov 1 , Natalia M. Nurullina 1 , Svetlana N. Tuntseva 1 , Tatyana L. Puchkova 1 , Yaroslav O. Mezhuev 2, 3 , Kharlampii E. Kharlampidi 1 , Sergey V. Kolesov 4
Affiliation
|
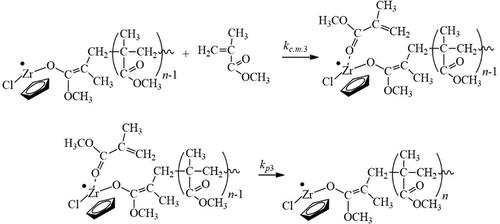
A kinetic model of the curing of methyl methacrylate adhesive (including nanocomposite methyl methacrylate adhesive) in the presence of the initiating systems “aryl peroxide + zirconocene dichloride” and “aryl hydroperoxide + zirconocene dichloride” is made. Computational experiments have been carried out which demonstrate the relationship of the curing rate with the curing temperature in the range of 323–343 K and with the ratio of the initial concentration of zirconocene dichloride to the initial concentration of the initiator [Mc]0/[I]0 for the following initiators: benzoyl peroxide (PB), ethylbenzene hydroperoxide (HPEB), and ethylbenzene hydroperoxide adduct with cadmium 2-ethyl hexanoate [HPEB·Cd(EH)2]. It is shown that in order to increase the curing rate of the adhesive, curing should be carried out at a higher temperature (343 K) and at a higher value of the ratio [Mc]0/[I]0 = 10 in the presence of the most rapidly decomposing initiator HPEB·Cd(EH)2. To increase the weight-average molecular weight of poly(methyl methacrylate), the proportion of syndiotactic triads in its composition, and consequently, to improve the adhesion strength and heat resistance of the adhesive joint, the curing of the adhesive must be carried out at the reduced temperature (323 K) and the reduced ratio of the [Mc]0/[I]0 = 0.1 in the presence of the least rapidly decomposing initiator HPEB.
中文翻译:

“过氧化物+二氯化二茂锆”引发体系存在下甲基丙烯酸甲酯胶粘剂固化时甲基丙烯酸甲酯聚合动力学
建立了“过氧化芳基+二氯化二茂锆”和“氢过氧化芳基+二氯化二茂锆”引发体系下甲基丙烯酸甲酯胶粘剂(包括纳米复合甲基丙烯酸甲酯胶粘剂)的固化动力学模型。进行了计算实验,证明了固化速率与 323-343 K 范围内的固化温度以及二氯化锆初始浓度与引发剂初始浓度之比的关系 [Mc] 0 /[I] 0 适用于以下引发剂:过氧化苯甲酰 (PB)、氢过氧化乙苯 (HPEB) 以及氢过氧化乙苯与 2-乙基己酸镉的加合物 [HPEB·Cd(EH) < b2>]。结果表明,为了提高胶粘剂的固化速率,应在较高的温度(343 K)和较高的[Mc] 0 /[I]比值下进行固化。在分解速度最快的引发剂 HPEB·Cd(EH) 2 存在下, 0 = 10。为了提高聚甲基丙烯酸甲酯的重均分子量、间规三元组在其组成中的比例,从而提高粘合接头的粘合强度和耐热性,粘合剂的固化必须在以下温度下进行:在分解速度最慢的引发剂 HPEB 存在下,降低温度 (323 K) 和 [Mc] 0 /[I] 0 = 0.1 的降低比率。
更新日期:2024-06-29
中文翻译:

“过氧化物+二氯化二茂锆”引发体系存在下甲基丙烯酸甲酯胶粘剂固化时甲基丙烯酸甲酯聚合动力学
建立了“过氧化芳基+二氯化二茂锆”和“氢过氧化芳基+二氯化二茂锆”引发体系下甲基丙烯酸甲酯胶粘剂(包括纳米复合甲基丙烯酸甲酯胶粘剂)的固化动力学模型。进行了计算实验,证明了固化速率与 323-343 K 范围内的固化温度以及二氯化锆初始浓度与引发剂初始浓度之比的关系 [Mc] 0 /[I] 0 适用于以下引发剂:过氧化苯甲酰 (PB)、氢过氧化乙苯 (HPEB) 以及氢过氧化乙苯与 2-乙基己酸镉的加合物 [HPEB·Cd(EH) < b2>]。结果表明,为了提高胶粘剂的固化速率,应在较高的温度(343 K)和较高的[Mc] 0 /[I]比值下进行固化。在分解速度最快的引发剂 HPEB·Cd(EH) 2 存在下, 0 = 10。为了提高聚甲基丙烯酸甲酯的重均分子量、间规三元组在其组成中的比例,从而提高粘合接头的粘合强度和耐热性,粘合剂的固化必须在以下温度下进行:在分解速度最慢的引发剂 HPEB 存在下,降低温度 (323 K) 和 [Mc] 0 /[I] 0 = 0.1 的降低比率。






































 京公网安备 11010802027423号
京公网安备 11010802027423号