当前位置:
X-MOL 学术
›
Redox Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lactate drives epithelial-mesenchymal transition in diabetic kidney disease via the H3K14la/KLF5 pathway
Redox Biology ( IF 10.7 ) Pub Date : 2024-06-20 , DOI: 10.1016/j.redox.2024.103246 Xuanxuan Zhang 1 , Jicong Chen 1 , Ruohui Lin 1 , Yaping Huang 1 , Ziyuan Wang 1 , Susu Xu 2 , Lei Wang 1 , Fang Chen 3 , Jian Zhang 4 , Ke Pan 1 , Zhiqi Yin 1
Redox Biology ( IF 10.7 ) Pub Date : 2024-06-20 , DOI: 10.1016/j.redox.2024.103246 Xuanxuan Zhang 1 , Jicong Chen 1 , Ruohui Lin 1 , Yaping Huang 1 , Ziyuan Wang 1 , Susu Xu 2 , Lei Wang 1 , Fang Chen 3 , Jian Zhang 4 , Ke Pan 1 , Zhiqi Yin 1
Affiliation
|
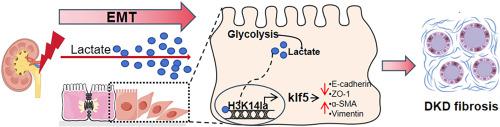
High levels of urinary lactate are an increased risk of progression in patients with diabetic kidney disease (DKD). However, it is still unveiled how lactate drive DKD. Epithelial-mesenchymal transition (EMT), which is characterized by the loss of epithelial cells polarity and cell-cell adhesion, and the acquisition of mesenchymal-like phenotypes, is widely recognized a critical contributor to DKD. Here, we found a switch from oxidative phosphorylation (OXPHOS) toward glycolysis in AGEs-induced renal tubular epithelial cells, thus leading to elevated levels of renal lactic acid. We demonstrated that reducing the lactate levels markedly delayed EMT progression and improved renal tubular fibrosis in DKD. Mechanically, we observed lactate increased the levels of histone H3 lysine 14 lactylation (H3K14la) in DKD. ChIP-seq & RNA-seq results showed histone lactylation contributed to EMT process by facilitating KLF5 expression. Moreover, KLF5 recognized the promotor of cdh1 and inhibited its transcription, which accelerated EMT of DKD. Additionally, nephro-specific knockdown and pharmacological inhibition of KLF5 diminished EMT development and attenuated DKD fibrosis. Thus, our study provides better understanding of epigenetic regulation of DKD pathogenesis, and new therapeutic strategy for DKD by disruption of the lactate-drived H3K14la/KLF5 pathway.
中文翻译:

乳酸通过 H3K14la/KLF5 途径驱动糖尿病肾病中的上皮间质转化
高水平的尿乳酸会增加糖尿病肾病 (DKD) 患者病情进展的风险。然而,乳酸如何驱动 DKD 仍不清楚。上皮-间质转化 (EMT) 的特点是上皮细胞极性和细胞间粘附力的丧失以及间质样表型的获得,被广泛认为是 DKD 的关键因素。在这里,我们发现 AGE 诱导的肾小管上皮细胞从氧化磷酸化 (OXPHOS) 转向糖酵解,从而导致肾乳酸水平升高。我们证明,降低乳酸水平可显着延缓 DKD 的 EMT 进展并改善肾小管纤维化。从机械角度来看,我们观察到乳酸增加了 DKD 中组蛋白 H3 赖氨酸 14 乳酰化 (H3K14la) 的水平。 ChIP-seq 和 RNA-seq 结果显示组蛋白乳酰化通过促进 KLF5 表达来促进 EMT 过程。此外,KLF5识别cdh1的启动子并抑制其转录,从而加速DKD的EMT。此外,KLF5 的肾特异性敲低和药理学抑制可减少 EMT 的发展并减轻 DKD 纤维化。因此,我们的研究提供了对 DKD 发病机制的表观遗传调控的更好理解,以及通过破坏乳酸驱动的 H3K14la/KLF5 通路来治疗 DKD 的新策略。
更新日期:2024-06-20
中文翻译:

乳酸通过 H3K14la/KLF5 途径驱动糖尿病肾病中的上皮间质转化
高水平的尿乳酸会增加糖尿病肾病 (DKD) 患者病情进展的风险。然而,乳酸如何驱动 DKD 仍不清楚。上皮-间质转化 (EMT) 的特点是上皮细胞极性和细胞间粘附力的丧失以及间质样表型的获得,被广泛认为是 DKD 的关键因素。在这里,我们发现 AGE 诱导的肾小管上皮细胞从氧化磷酸化 (OXPHOS) 转向糖酵解,从而导致肾乳酸水平升高。我们证明,降低乳酸水平可显着延缓 DKD 的 EMT 进展并改善肾小管纤维化。从机械角度来看,我们观察到乳酸增加了 DKD 中组蛋白 H3 赖氨酸 14 乳酰化 (H3K14la) 的水平。 ChIP-seq 和 RNA-seq 结果显示组蛋白乳酰化通过促进 KLF5 表达来促进 EMT 过程。此外,KLF5识别cdh1的启动子并抑制其转录,从而加速DKD的EMT。此外,KLF5 的肾特异性敲低和药理学抑制可减少 EMT 的发展并减轻 DKD 纤维化。因此,我们的研究提供了对 DKD 发病机制的表观遗传调控的更好理解,以及通过破坏乳酸驱动的 H3K14la/KLF5 通路来治疗 DKD 的新策略。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号