当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical reduction processes and extraction of titanium with different valence states in molten CaO-CaF2 slags
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2024-06-27 , DOI: 10.1016/j.jallcom.2024.175379 Liqi Zhang , Xu Zhang , Bowen Huang , Yusheng Yang , Zengwu Zhao
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2024-06-27 , DOI: 10.1016/j.jallcom.2024.175379 Liqi Zhang , Xu Zhang , Bowen Huang , Yusheng Yang , Zengwu Zhao
|
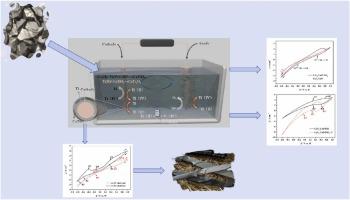
Titanium is an excellent structural and functional material. At present, the production of titanium has long process cycle, high energy consumption, serious pollution and other characteristics, so the development of low-cost titanium production methods has extremely far-reaching significance. In this work, the electrochemical reduction process of Ti(IV) and Ti(II) in the CaF-CaO melt were investigated by cyclic voltammetry and square wave voltammetry measurements at 1740 K, respectively. In the CaF-CaO-5 wt% TiO melt, Ti(IV) ions were reduced to Ti by one step. One measure was taken to lower the valence state of Ti ions: Ti(IV) ions in the melt was changed to Ti(II) by adding Ti. Ti(IV) ions were reduced in two steps: in the CaF-CaO-5 wt% TiO-Ti melt, Ti(IV) → Ti(II) and Ti(II) → Ti, respectively, and in the CaF-CaO-5 wt% TiO, the Ti(II) ions were reduced in a single step: Ti(II) → Ti. It was found that both migration and mass transfer affected the reduction processes. In contrast to the Ti(IV) reduction to Ti process, the Ti(II) reduction to Ti process showed a higher diffusion rate. Electrolytes and electrodes were analyzed after titanium electrodeposition by XRD. It was found that there were no metal titanium was detected in the electrolyte placed close to the cathode after electrodeposition. It believed that the formed metallic Ti reacted with dissolved O in the melt to form TiO. The reduction process of Ti(IV) on the iron electrode was investigated, the metal Ti was reduced by alloying process, the oxidation of Ti was hindered, there was a dense structure layer of CaTiO outside the reduced metal Ti that prevented the ions transfer and affected the Ti(IV) ions migration process, which might be the main factor that the Ti(IV) reduction process was controlled by migration. The lower valence state of Ti in the melt promoted the formation of CaTiO, which increased the ion migration rate and decreased the mass transfer rate. It was suggested that titanium or titanium alloys may be prepared by reducing the valence of Ti(IV) in minerals and altering the melt composition. It is feasible to prepare titanium and titanium alloys by MOE short process using ore as raw material.
中文翻译:

熔融CaO-CaF2渣中不同价态钛的电化学还原过程及提取
钛是一种优良的结构和功能材料。目前钛的生产具有工艺周期长、能耗高、污染严重等特点,因此发展低成本的钛生产方法具有极其深远的意义。本工作分别在1740K下通过循环伏安法和方波伏安法测量了CaF-CaO熔体中Ti(IV)和Ti(II)的电化学还原过程。在CaF-CaO-5wt% TiO熔体中,Ti(IV)离子一步被还原为Ti。采取一种降低Ti离子价态的措施:通过添加Ti将熔体中的Ti(IV)离子转变为Ti(II)离子。 Ti(IV)离子分两步被还原:在CaF-CaO-5wt% TiO-Ti熔体中,Ti(IV)→Ti(II)和Ti(II)→Ti,分别,在CaF-CaO中-5wt% TiO,Ti(II) 离子一步被还原:Ti(II) → Ti。研究发现迁移和传质都会影响还原过程。与Ti(IV)还原成Ti过程相比,Ti(II)还原成Ti过程表现出更高的扩散速率。钛电沉积后通过 XRD 分析电解质和电极。结果发现,电沉积后靠近阴极放置的电解液中没有检测到金属钛。据认为,形成的金属Ti与熔体中溶解的O反应形成TiO。 研究了Ti(IV)在铁电极上的还原过程,金属Ti通过合金化过程被还原,Ti的氧化受到阻碍,被还原的金属Ti外有一层致密的CaTiO结构层,阻碍了离子的迁移和迁移。影响Ti(IV)离子迁移过程,这可能是Ti(IV)还原过程受迁移控制的主要因素。熔体中Ti的较低价态促进了CaTiO的形成,从而增加了离子迁移速率并降低了传质速率。有人提出,可以通过降低矿物中Ti(IV)的价态并改变熔体成分来制备钛或钛合金。以矿石为原料,采用MOE短流程制备钛及钛合金是可行的。
更新日期:2024-06-27
中文翻译:

熔融CaO-CaF2渣中不同价态钛的电化学还原过程及提取
钛是一种优良的结构和功能材料。目前钛的生产具有工艺周期长、能耗高、污染严重等特点,因此发展低成本的钛生产方法具有极其深远的意义。本工作分别在1740K下通过循环伏安法和方波伏安法测量了CaF-CaO熔体中Ti(IV)和Ti(II)的电化学还原过程。在CaF-CaO-5wt% TiO熔体中,Ti(IV)离子一步被还原为Ti。采取一种降低Ti离子价态的措施:通过添加Ti将熔体中的Ti(IV)离子转变为Ti(II)离子。 Ti(IV)离子分两步被还原:在CaF-CaO-5wt% TiO-Ti熔体中,Ti(IV)→Ti(II)和Ti(II)→Ti,分别,在CaF-CaO中-5wt% TiO,Ti(II) 离子一步被还原:Ti(II) → Ti。研究发现迁移和传质都会影响还原过程。与Ti(IV)还原成Ti过程相比,Ti(II)还原成Ti过程表现出更高的扩散速率。钛电沉积后通过 XRD 分析电解质和电极。结果发现,电沉积后靠近阴极放置的电解液中没有检测到金属钛。据认为,形成的金属Ti与熔体中溶解的O反应形成TiO。 研究了Ti(IV)在铁电极上的还原过程,金属Ti通过合金化过程被还原,Ti的氧化受到阻碍,被还原的金属Ti外有一层致密的CaTiO结构层,阻碍了离子的迁移和迁移。影响Ti(IV)离子迁移过程,这可能是Ti(IV)还原过程受迁移控制的主要因素。熔体中Ti的较低价态促进了CaTiO的形成,从而增加了离子迁移速率并降低了传质速率。有人提出,可以通过降低矿物中Ti(IV)的价态并改变熔体成分来制备钛或钛合金。以矿石为原料,采用MOE短流程制备钛及钛合金是可行的。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号