当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoinduced [4 + 2]-cycloaddition reactions of vinyldiazo compounds for the construction of heterocyclic and bicyclic rings
Chemical Science ( IF 7.6 ) Pub Date : 2024-06-28 , DOI: 10.1039/d4sc03558e Michael P Doyle , Ming Bao , Arnold R. R. Bohórquez , Hadi Arman
Chemical Science ( IF 7.6 ) Pub Date : 2024-06-28 , DOI: 10.1039/d4sc03558e Michael P Doyle , Ming Bao , Arnold R. R. Bohórquez , Hadi Arman
|
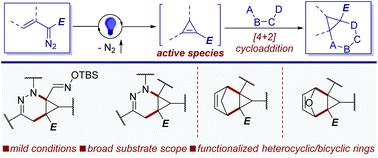
Highly selective formal [4 + 2]-cycloaddition of vinyldiazoacetates with azoalkenes from α-halohydrazones, as well as with cyclopentadiene and furan, occurs with light irradiation at room temperature, producing highly functionalized heterocyclic and bicyclic compounds in good yields and excellent diastereoseletivity. Under blue light these vinyldiazoacetate reagents selectively form unstable cyclopropenes that undergo intermolecular cycloaddition reactions at a faster rate than their competitive ene dimerization. [4 + 2]-cycloaddition of vinyldiazoacetates with in situ formed azoalkenes produces bicyclo[4.1.0]tetrahydropyridazine derivatives and, together with their cycloaddition using cyclopentadiene and furan that form tricyclic compounds, they occur with high chemoselectivity and diastereocontrol, good functional group tolerance, and excellent scalability. Subsequent transformations portray the synthetic versatility of these structures.
中文翻译:

乙烯基重氮化合物的光诱导[4 + 2]-环加成反应用于构建杂环和双环
在室温光照射下,重氮乙酸乙烯酯与来自 α-卤代腙的偶氮烯烃以及环戊二烯和呋喃发生高度选择性的形式 [4 + 2]-环加成,产生高产率和优异非对映选择性的高度官能化的杂环和双环化合物。在蓝光下,这些重氮乙酸乙烯酯试剂选择性地形成不稳定的环丙烯,其以比其竞争性烯二聚化更快的速率进行分子间环加成反应。乙烯基重氮乙酸酯与原位形成的偶氮烯烃发生[4 + 2]-环加成反应生成双环[4.1.0]四氢哒嗪衍生物,并且与使用环戊二烯和呋喃形成三环化合物的环加成反应一起,它们具有高化学选择性和非对映控制性以及良好的官能团耐受性,以及出色的可扩展性。随后的转变描绘了这些结构的综合多功能性。
更新日期:2024-07-03
中文翻译:

乙烯基重氮化合物的光诱导[4 + 2]-环加成反应用于构建杂环和双环
在室温光照射下,重氮乙酸乙烯酯与来自 α-卤代腙的偶氮烯烃以及环戊二烯和呋喃发生高度选择性的形式 [4 + 2]-环加成,产生高产率和优异非对映选择性的高度官能化的杂环和双环化合物。在蓝光下,这些重氮乙酸乙烯酯试剂选择性地形成不稳定的环丙烯,其以比其竞争性烯二聚化更快的速率进行分子间环加成反应。乙烯基重氮乙酸酯与原位形成的偶氮烯烃发生[4 + 2]-环加成反应生成双环[4.1.0]四氢哒嗪衍生物,并且与使用环戊二烯和呋喃形成三环化合物的环加成反应一起,它们具有高化学选择性和非对映控制性以及良好的官能团耐受性,以及出色的可扩展性。随后的转变描绘了这些结构的综合多功能性。






































 京公网安备 11010802027423号
京公网安备 11010802027423号