当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Process for the Remediation of Titanogypsum (Red Gypsum) Using Weak Acid and CaCl2 to Produce Saleable α-Gypsum and FeCl2·4H2O
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-27 , DOI: 10.1021/acs.iecr.4c01190 Shuaifeng Liu 1, 2 , Edouard Asselin 3 , Zhibao Li 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-27 , DOI: 10.1021/acs.iecr.4c01190 Shuaifeng Liu 1, 2 , Edouard Asselin 3 , Zhibao Li 1
Affiliation
|
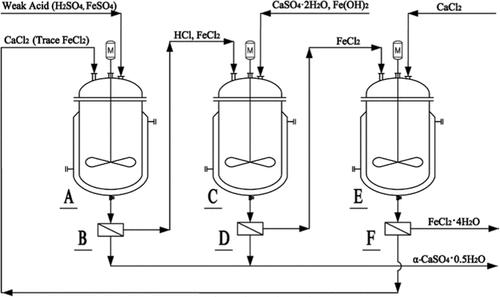
The disposal of titanogypsum, also known as red gypsum, which is generated during the titanium dioxide sulfate process, has become a significant environmental challenge. We propose a new process to recover iron impurities such as FeCl2·4H2O and to convert titanogypsum to saleable hemihydrate gypsum. For this purpose, we use HCl–FeCl2 solution generated via the reaction of H2SO4–FeSO4 with CaCl2 to leach iron hydroxide from titanogypsum. The hemihydrate precipitation and phase-transition kinetics of the reaction of HCl–H2SO4–FeSO4 with CaCl2 are experimentally investigated at 353 K. The common ion effect of CaCl2 in the FeCl2 solution for FeCl2·4H2O crystallization is predicted by NRTL modeling of the solid–liquid equilibria of the FeCl2–CaCl2–H2O system. High-quality, saleable α-gypsum and FeCl2·4H2O solids obtained by treating titanogypsum samples provided by a commercial processing plant further shows that the new process is feasible.
中文翻译:

利用弱酸和CaCl2修复钛石膏(红石膏)生产可销售的α-石膏和FeCl2·4H2O的工艺
在二氧化钛硫酸盐工艺过程中产生的钛石膏(也称为红石膏)的处置已成为一项重大的环境挑战。我们提出了一种新工艺来回收 FeCl 2 ·4H 2 O 等铁杂质,并将钛石膏转化为可销售的半水石膏。为此,我们使用 H 2 SO 4 –FeSO 4 与 CaCl < 反应生成的 HCl–FeCl 2 溶液。 b6>从钛石膏中浸出氢氧化铁。 HCl–H 2 SO 4 –FeSO 4 与 CaCl 2 反应的半水合物沉淀和相变动力学实验在 353 K 下进行了研究。CaCl 2 在 FeCl 2 溶液中对 FeCl 2 ·4H 2 O 结晶的共同离子效应为通过 FeCl 2 –CaCl 2 –H 2 O 系统固液平衡的 NRTL 模型进行预测。通过处理商业加工厂提供的钛石膏样品获得高质量、可销售的α-石膏和FeCl 2 ·4H 2 O固体,进一步表明新工艺是可行的。
更新日期:2024-06-27
中文翻译:

利用弱酸和CaCl2修复钛石膏(红石膏)生产可销售的α-石膏和FeCl2·4H2O的工艺
在二氧化钛硫酸盐工艺过程中产生的钛石膏(也称为红石膏)的处置已成为一项重大的环境挑战。我们提出了一种新工艺来回收 FeCl 2 ·4H 2 O 等铁杂质,并将钛石膏转化为可销售的半水石膏。为此,我们使用 H 2 SO 4 –FeSO 4 与 CaCl < 反应生成的 HCl–FeCl 2 溶液。 b6>从钛石膏中浸出氢氧化铁。 HCl–H 2 SO 4 –FeSO 4 与 CaCl 2 反应的半水合物沉淀和相变动力学实验在 353 K 下进行了研究。CaCl 2 在 FeCl 2 溶液中对 FeCl 2 ·4H 2 O 结晶的共同离子效应为通过 FeCl 2 –CaCl 2 –H 2 O 系统固液平衡的 NRTL 模型进行预测。通过处理商业加工厂提供的钛石膏样品获得高质量、可销售的α-石膏和FeCl 2 ·4H 2 O固体,进一步表明新工艺是可行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号