当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Investigation of Oxidative Methanol Carbonylation with Mixed Cu(I)/Cu(II)–N-Methylimidazole Catalysts
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-24 , DOI: 10.1021/acs.iecr.4c01210 Guoxin Li 1 , Shiwei Wang 2 , Xingmin Liu 2 , Zicheng Wen 1 , Shouying Huang 1 , Jing Lv 1 , Xinbin Ma 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-24 , DOI: 10.1021/acs.iecr.4c01210 Guoxin Li 1 , Shiwei Wang 2 , Xingmin Liu 2 , Zicheng Wen 1 , Shouying Huang 1 , Jing Lv 1 , Xinbin Ma 1
Affiliation
|
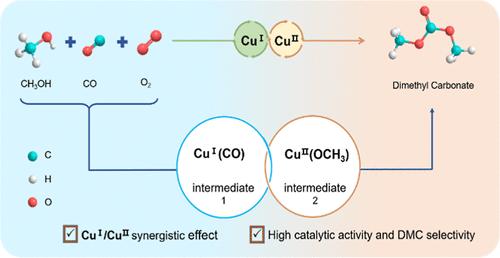
Copper complex catalysts exhibit excellent activity for the oxidative carbonylation of methanol to produce dimethyl carbonate (DMC). However, the reaction mechanism over Cu(I) or Cu(II) complex catalysts is not fully understood. In this study, homogeneous CuCl/CuCl2–NMI-m/n (N = N-methylimidazole; m/n = molar ratio of Cu(I) and Cu(II)) catalysts with organic ligand coordination are designed for oxidative carbonylation. The optimized CuCl/CuCl2–NMI-0.3/0.7 catalyst showed a TOF as high as 3.4 h–1 and a DMC selectivity of 67.2% based on oxygen, which were superior to those of CuCl–NMI or CuCl2–NMI. Our experiments suggested that the high activity and selectivity were assigned to the synergistic effect between Cu(I) and Cu(II), in which Cu(I) and Cu(II) are mainly responsible for the rapid generation of copper carbonyl and copper methoxy intermediates, respectively. This synergistic effect not only enhances the reaction activity but also ensures high DMC selectivity.
中文翻译:

Cu(I)/Cu(II)–N-甲基咪唑混合催化剂氧化甲醇羰基化反应的机理研究
铜络合物催化剂对于甲醇氧化羰基化生产碳酸二甲酯(DMC)表现出优异的活性。然而,Cu(I) 或 Cu(II) 络合物催化剂的反应机理尚不完全清楚。本研究中,采用有机配体配位的均相 CuCl/CuCl 2 –NMI-m/n(N = N-甲基咪唑;m/n = Cu(I) 和 Cu(II) 的摩尔比)催化剂设计用于氧化羰基化。优化后的CuCl/CuCl 2 –NMI-0.3/0.7催化剂的TOF高达3.4 h –1 ,基于氧的DMC选择性为67.2%,优于现有催化剂。 CuCl–NMI 或 CuCl 2 –NMI。我们的实验表明,高活性和选择性归因于 Cu(I) 和 Cu(II) 之间的协同作用,其中 Cu(I) 和 Cu(II) 主要负责快速生成羰基铜和甲氧基铜分别为中间体。这种协同效应不仅提高了反应活性,而且保证了DMC的高选择性。
更新日期:2024-06-24
中文翻译:

Cu(I)/Cu(II)–N-甲基咪唑混合催化剂氧化甲醇羰基化反应的机理研究
铜络合物催化剂对于甲醇氧化羰基化生产碳酸二甲酯(DMC)表现出优异的活性。然而,Cu(I) 或 Cu(II) 络合物催化剂的反应机理尚不完全清楚。本研究中,采用有机配体配位的均相 CuCl/CuCl 2 –NMI-m/n(N = N-甲基咪唑;m/n = Cu(I) 和 Cu(II) 的摩尔比)催化剂设计用于氧化羰基化。优化后的CuCl/CuCl 2 –NMI-0.3/0.7催化剂的TOF高达3.4 h –1 ,基于氧的DMC选择性为67.2%,优于现有催化剂。 CuCl–NMI 或 CuCl 2 –NMI。我们的实验表明,高活性和选择性归因于 Cu(I) 和 Cu(II) 之间的协同作用,其中 Cu(I) 和 Cu(II) 主要负责快速生成羰基铜和甲氧基铜分别为中间体。这种协同效应不仅提高了反应活性,而且保证了DMC的高选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号