当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhanced Catalytic Oxidation Reactivity over Atomically Dispersed Pt/CeO2 Catalysts by CO Activation
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-06-27 , DOI: 10.1021/acs.est.4c02022 Zihao Li 1 , Zhisong Liu 1 , Guanqun Gao 1 , Weina Zhao 2 , Yongjun Jiang 3 , Xuan Tang 3 , Sheng Dai 3 , Zan Qu 1 , Naiqiang Yan 1 , Lei Ma 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-06-27 , DOI: 10.1021/acs.est.4c02022 Zihao Li 1 , Zhisong Liu 1 , Guanqun Gao 1 , Weina Zhao 2 , Yongjun Jiang 3 , Xuan Tang 3 , Sheng Dai 3 , Zan Qu 1 , Naiqiang Yan 1 , Lei Ma 1
Affiliation
|
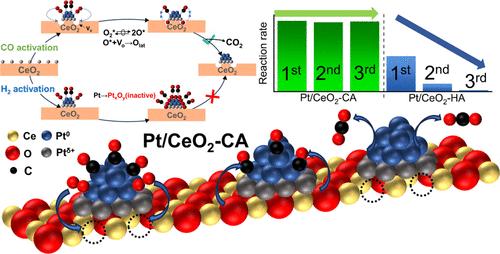
The elevation of the low-temperature oxidation activity for Pt/CeO2 catalysts is challenging to meet the increasingly stringent requirements for effectively eliminating carbon monoxide (CO) from automobile exhaust. Although reducing activation is a facile strategy for boosting reactivity, past research has mainly concentrated on applying H2 as the reductant, ignoring the reduction capabilities of CO itself, a prevalent component of automobile exhaust. Herein, atomically dispersed Pt/CeO2 was fabricated and activated by CO, which could lower the 90% conversion temperature (T90) by 256 °C and achieve a 20-fold higher CO consumption rate at 200 °C. The activated Pt/CeO2 catalysts showed exceptional catalytic oxidation activity and robust hydrothermal stability under the simulated working conditions for gasoline or diesel exhausts. Characterization results illustrated that the CO activation triggered the formation of a large portion of Pt0 terrace sites, acting as inherent active sites for CO oxidation. Besides, CO activation weakened the Pt–O–Ce bond strength to generate a surface oxygen vacancy (Vo). It served as the oxygen reservoir to store the dissociated oxygen and convert it into active dioxygen intermediates. Conversely, H2 activation failed to stimulate Vo, but triggered a deactivating transformation of the Pt nanocluster into inactive PtxOy in the presence of oxygen. The present work offers coherent insight into the upsurging effect of CO activation on Pt/CeO2, aiming to set up a valuable avenue in elevating the efficiency of eliminating CO, C3H6, and NH3 from automobile exhaust.
中文翻译:

通过 CO 活化增强原子分散 Pt/CeO2 催化剂的催化氧化反应活性
为了满足有效消除汽车尾气中一氧化碳(CO)日益严格的要求,提高Pt/CeO 2 催化剂的低温氧化活性具有挑战性。尽管减少活化是提高反应活性的一种简便策略,但过去的研究主要集中在应用H 2 作为还原剂,忽略了汽车尾气中常见成分CO 本身的还原能力。在此,制备了原子分散的Pt/CeO 2 并通过CO活化,可以将90%的转化温度(T 90 )降低256°C,并实现20倍的高转化率。 200 °C 时的 CO 消耗率。活化的Pt/CeO 2 催化剂在模拟汽油或柴油废气的工况下表现出优异的催化氧化活性和强大的水热稳定性。表征结果表明,CO 活化引发了大部分 Pt 0 阶地位点的形成,作为 CO 氧化的固有活性位点。此外,CO活化削弱了Pt-O-Ce键强度,产生表面氧空位(V o )。它充当氧气储存库,储存离解的氧气并将其转化为活性分子氧中间体。相反,H 2 激活未能刺激 V o ,但引发 Pt 纳米团簇失活转变为不活跃的 Pt x O y 在氧气存在下。目前的工作对 CO 活化对 Pt/CeO 2 的上升效应提供了一致的见解,旨在为提高消除 CO、C 3 H < 的效率建立一个有价值的途径。 b13> 和 NH 3 来自汽车尾气。
更新日期:2024-06-27
中文翻译:

通过 CO 活化增强原子分散 Pt/CeO2 催化剂的催化氧化反应活性
为了满足有效消除汽车尾气中一氧化碳(CO)日益严格的要求,提高Pt/CeO 2 催化剂的低温氧化活性具有挑战性。尽管减少活化是提高反应活性的一种简便策略,但过去的研究主要集中在应用H 2 作为还原剂,忽略了汽车尾气中常见成分CO 本身的还原能力。在此,制备了原子分散的Pt/CeO 2 并通过CO活化,可以将90%的转化温度(T 90 )降低256°C,并实现20倍的高转化率。 200 °C 时的 CO 消耗率。活化的Pt/CeO 2 催化剂在模拟汽油或柴油废气的工况下表现出优异的催化氧化活性和强大的水热稳定性。表征结果表明,CO 活化引发了大部分 Pt 0 阶地位点的形成,作为 CO 氧化的固有活性位点。此外,CO活化削弱了Pt-O-Ce键强度,产生表面氧空位(V o )。它充当氧气储存库,储存离解的氧气并将其转化为活性分子氧中间体。相反,H 2 激活未能刺激 V o ,但引发 Pt 纳米团簇失活转变为不活跃的 Pt x O y 在氧气存在下。目前的工作对 CO 活化对 Pt/CeO 2 的上升效应提供了一致的见解,旨在为提高消除 CO、C 3 H < 的效率建立一个有价值的途径。 b13> 和 NH 3 来自汽车尾气。











































 京公网安备 11010802027423号
京公网安备 11010802027423号