当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photocatalytic Reduction of Perrhenate and Pertechnetate in a Strongly Acidic Aqueous Solution
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-06-27 , DOI: 10.1021/acs.est.4c02511 Heng Yang 1 , Hao Deng 1 , Pengliang Liang 2 , XianJin Ma 1 , Jing Yin 1 , Long Jiang 1 , Yanyan Chen 1 , Shuying Shi 1 , Huiqiang Liu 1 , Xue Ma 1 , Yuxiang Li 1 , Ying Xiong 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-06-27 , DOI: 10.1021/acs.est.4c02511 Heng Yang 1 , Hao Deng 1 , Pengliang Liang 2 , XianJin Ma 1 , Jing Yin 1 , Long Jiang 1 , Yanyan Chen 1 , Shuying Shi 1 , Huiqiang Liu 1 , Xue Ma 1 , Yuxiang Li 1 , Ying Xiong 1
Affiliation
|
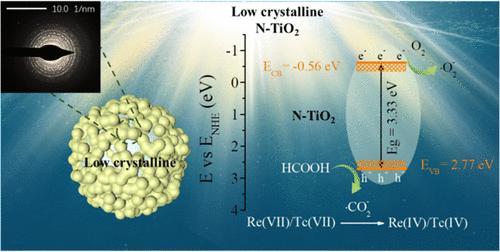
Pertechnetate (99TcO4–), a physiologically toxic radioactive anion, is of great concern due to its high mobility in environmental contamination remediation. Although the soluble oxyanion can be photoreduced to sparingly soluble TcO2·nH2O, its effective removal from a strongly acidic aqueous solution remains a challenge. Here, we found that low-crystalline nitrogen-doped titanium oxide (N-TiO2, 0.6 g L–1) could effectively uptake perrhenate (ReO4–, 10 mg L–1, a nonradioactive surrogate for TcO4–) with 50.8% during 360 min under simulated sunlight irradiation at pH 1.0, but P25 and anatase could not. The nitrogen active center formed by trace nitrogen doping in N-TiO2 can promote the separation and transfer of photogenerated carriers. The positive valence band value of N-TiO2 is slightly higher than those of P25 and anatase, which means that the photogenerated holes have a stronger oxidizability. These holes are involved in the formation of strong reducing •CO2– radicals from formic acid oxidation. The active radicals convert ReO4– to Re(VI), which is subsequently disproportionated to Re(IV) and Re(VII). Effective photocatalytic reduction/removal of Re(VII)/Tc(VII) is performed on the material, which may be considered a potential and convenient strategy for technetium decontamination and extraction in a strongly acidic aqueous solution.
中文翻译:

强酸性水溶液中高铼酸盐和高锝酸盐的光催化还原
高锝酸( 99 TcO 4 – )是一种具有生理毒性的放射性阴离子,由于其在环境污染修复中的高流动性而备受关注。尽管可溶性氧阴离子可以光还原为微溶性TcO 2 ·nH 2 O,但其从强酸性水溶液中的有效去除仍然是一个挑战。在这里,我们发现低结晶氮掺杂二氧化钛(N-TiO 2 , 0.6 g L –1 )可以有效地吸收高铼酸(ReO 4 < b8> , 10 mg·L –1 ,TcO 的非放射性替代品 4 – ) 在 pH 值模拟阳光照射下 360 分钟内具有 50.8% 1.0,但 P25 和锐钛矿不能。 N-TiO 2 中微量氮掺杂形成的氮活性中心可以促进光生载流子的分离和转移。 N-TiO 2 的正价带值略高于P25和锐钛矿,这意味着光生空穴具有更强的氧化性。这些空穴参与甲酸氧化过程中强还原性 • CO 2 – 自由基的形成。活性自由基将 ReO 4 – 转化为 Re(VI),随后歧化为 Re(IV) 和 Re(VII)。对该材料进行了有效的光催化还原/去除Re(VII)/Tc(VII),这可能被认为是强酸性水溶液中锝净化和萃取的潜在且方便的策略。
更新日期:2024-06-27
中文翻译:

强酸性水溶液中高铼酸盐和高锝酸盐的光催化还原
高锝酸( 99 TcO 4 – )是一种具有生理毒性的放射性阴离子,由于其在环境污染修复中的高流动性而备受关注。尽管可溶性氧阴离子可以光还原为微溶性TcO 2 ·nH 2 O,但其从强酸性水溶液中的有效去除仍然是一个挑战。在这里,我们发现低结晶氮掺杂二氧化钛(N-TiO 2 , 0.6 g L –1 )可以有效地吸收高铼酸(ReO 4 < b8> , 10 mg·L –1 ,TcO 的非放射性替代品 4 – ) 在 pH 值模拟阳光照射下 360 分钟内具有 50.8% 1.0,但 P25 和锐钛矿不能。 N-TiO 2 中微量氮掺杂形成的氮活性中心可以促进光生载流子的分离和转移。 N-TiO 2 的正价带值略高于P25和锐钛矿,这意味着光生空穴具有更强的氧化性。这些空穴参与甲酸氧化过程中强还原性 • CO 2 – 自由基的形成。活性自由基将 ReO 4 – 转化为 Re(VI),随后歧化为 Re(IV) 和 Re(VII)。对该材料进行了有效的光催化还原/去除Re(VII)/Tc(VII),这可能被认为是强酸性水溶液中锝净化和萃取的潜在且方便的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号