当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the Adsorption Mechanism of Rhodamine B onto Conjugated Polymeric Nanoparticles Using Isothermal Calorimetry and Monte Carlo Simulations
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-06-26 , DOI: 10.1021/acs.jpcc.4c01624 Rodrigo A. Ponzio , Emmanuel Odella , Elise Prost 1 , Raquel Gutiérrez-Climente 2 , Rodrigo E. Palacios , Luminita Duma 3 , Carlos A. Chesta
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-06-26 , DOI: 10.1021/acs.jpcc.4c01624 Rodrigo A. Ponzio , Emmanuel Odella , Elise Prost 1 , Raquel Gutiérrez-Climente 2 , Rodrigo E. Palacios , Luminita Duma 3 , Carlos A. Chesta
Affiliation
|
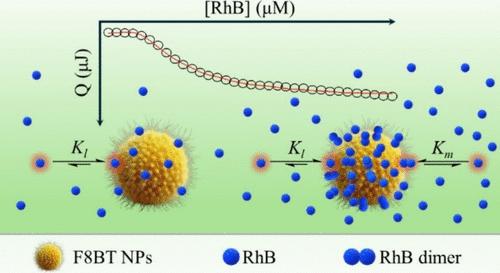
We present herein a comprehensive study on the thermodynamics of rhodamine B (RhB) adsorption onto poly(9,9-dioctylfluorene-alt-benzothiadiazole) (F8BT) nanoparticles (NPs) dispersed in water using the isothermal calorimetric titration (ITC) technique. ITC experiments were carried out over a wide temperature range (5–65 °C), and the resulting thermograms were subjected to a detailed thermodynamic analysis using different adsorption models. For this purpose, we developed a versatile computational code based on the Monte Carlo method. We found that the thermograms obtained at the different temperatures cannot be interpreted using the Langmuir, bi-Langmuir, or Brunauer, Emmet, Teller (BET) models. However, excellent thermogram fits were attained by applying the Langmuir model coupled with the formation of a RhB dimer on the surface of F8BT NPs. Using this model, the best fit to the experimental data at 25 °C yielded the following parameters for direct RhB adsorption on the F8BT NPs surface: equilibrium constant (Kl) of ∼2 × 107 M–1, enthalpy change (ΔHl) of ∼1 kJ mol–1, and entropy change (ΔSl) of ∼140 J K–1 mol–1. These results indicate that dye adsorption is predominantly an entropy-controlled process. The fit also provided parameters associated with the formation of a second dye adsorption layer, i.e., the formation of the RhB dimer on the F8BT NPs surface, revealing values of ∼1.7 × 105 M–1, ca. −20 kJ mol–1, and ∼30 J K–1 mol–1 for Km, ΔHm, and ΔSm, respectively. The latter two parameters are similar to those previously reported for RhB dimer formation in water (ΔHd = −12 kJ mol–1 and ΔSd = 22 J K–1 mol–1) supporting the chosen Langmuir-dimer model. The analysis of the thermograms also suggests that the NPs surface available for RhB adsorption is small, as compared to the total surface, and that it decreases with decreasing temperature. The latter observation is intriguing, especially since the F8BT NPs hydrodynamic diameter shows only a small decrease with decreasing temperature. To explain these results, we propose that the surface of the NPs harbors highly hydrated polar groups that reduce the accessibility of the dye for adsorption.
中文翻译:

利用等温量热法和蒙特卡罗模拟揭示罗丹明 B 在共轭聚合物纳米粒子上的吸附机制
我们在此提出了使用等温量热滴定 (ITC) 技术对分散在水中的聚(9,9-二辛基芴-替代苯并噻二唑) (F8BT) 纳米颗粒 (NP) 上罗丹明 B (RhB) 吸附的热力学的全面研究。 ITC 实验在较宽的温度范围(5–65 °C)下进行,并使用不同的吸附模型对所得热分析图进行详细的热力学分析。为此,我们开发了基于蒙特卡罗方法的通用计算代码。我们发现在不同温度下获得的热分析图无法使用 Langmuir、bi-Langmuir 或 Brunauer、Emmet、Teller (BET) 模型进行解释。然而,通过应用 Langmuir 模型并在 F8BT 纳米颗粒表面形成 RhB 二聚体,获得了出色的热分析图拟合。使用该模型,与 25 °C 下的实验数据最佳拟合,得到了在 F8BT 纳米颗粒表面直接吸附 RhB 的以下参数:平衡常数 (K) 为 ∼2 × 10 7 M –1 ,焓变 (ΔH) 为 ∼1 kJ mol –1 ,熵变 (ΔS) 为 ∼140 J K –1 mol –1 。这些结果表明染料吸附主要是一个熵控制的过程。该拟合还提供了与第二个染料吸附层的形成相关的参数,即 F8BT NP 表面上 RhB 二聚体的形成,揭示了 ∼1.7 × 10 5 M –1 和 ∼30 J K –1 mol –1 对于 K m 、ΔH m ,和 ΔS m ,分别。 后两个参数与之前报道的水中 RhB 二聚体形成的参数类似(ΔH d = -12 kJ mol –1 和 ΔS d = 22 J K < b16> mol –1 ) 支持所选的朗缪尔二聚体模型。热分析图还表明,与总表面相比,可供 RhB 吸附的纳米粒子表面较小,并且随着温度的降低而减小。后一个观察结果很有趣,特别是因为 F8BT 纳米粒子的流体动力学直径仅随着温度的降低而略有减小。为了解释这些结果,我们提出纳米颗粒的表面含有高度水合的极性基团,从而降低了染料吸附的可及性。
更新日期:2024-06-26
中文翻译:

利用等温量热法和蒙特卡罗模拟揭示罗丹明 B 在共轭聚合物纳米粒子上的吸附机制
我们在此提出了使用等温量热滴定 (ITC) 技术对分散在水中的聚(9,9-二辛基芴-替代苯并噻二唑) (F8BT) 纳米颗粒 (NP) 上罗丹明 B (RhB) 吸附的热力学的全面研究。 ITC 实验在较宽的温度范围(5–65 °C)下进行,并使用不同的吸附模型对所得热分析图进行详细的热力学分析。为此,我们开发了基于蒙特卡罗方法的通用计算代码。我们发现在不同温度下获得的热分析图无法使用 Langmuir、bi-Langmuir 或 Brunauer、Emmet、Teller (BET) 模型进行解释。然而,通过应用 Langmuir 模型并在 F8BT 纳米颗粒表面形成 RhB 二聚体,获得了出色的热分析图拟合。使用该模型,与 25 °C 下的实验数据最佳拟合,得到了在 F8BT 纳米颗粒表面直接吸附 RhB 的以下参数:平衡常数 (K) 为 ∼2 × 10 7 M –1 ,焓变 (ΔH) 为 ∼1 kJ mol –1 ,熵变 (ΔS) 为 ∼140 J K –1 mol –1 。这些结果表明染料吸附主要是一个熵控制的过程。该拟合还提供了与第二个染料吸附层的形成相关的参数,即 F8BT NP 表面上 RhB 二聚体的形成,揭示了 ∼1.7 × 10 5 M –1 和 ∼30 J K –1 mol –1 对于 K m 、ΔH m ,和 ΔS m ,分别。 后两个参数与之前报道的水中 RhB 二聚体形成的参数类似(ΔH d = -12 kJ mol –1 和 ΔS d = 22 J K < b16> mol –1 ) 支持所选的朗缪尔二聚体模型。热分析图还表明,与总表面相比,可供 RhB 吸附的纳米粒子表面较小,并且随着温度的降低而减小。后一个观察结果很有趣,特别是因为 F8BT 纳米粒子的流体动力学直径仅随着温度的降低而略有减小。为了解释这些结果,我们提出纳米颗粒的表面含有高度水合的极性基团,从而降低了染料吸附的可及性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号