当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Crystal Structure on the Aggregation of CdS Quantum Dots: Consequences for Photophysical Properties and Photocatalytic Hydrogen Evolution Activity
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-06-26 , DOI: 10.1021/acs.jpcc.4c02510 Vinícius Alevato 1 , Daniel Streater 2 , Jier Huang 3 , Stephanie Brock 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-06-26 , DOI: 10.1021/acs.jpcc.4c02510 Vinícius Alevato 1 , Daniel Streater 2 , Jier Huang 3 , Stephanie Brock 1
Affiliation
|
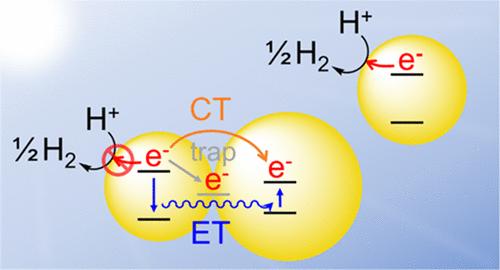
In the current pursuit of renewable energy resources, CdS emerges as a promising component for hydrogen production by solar-driven photocatalytic water splitting owing to its 2.4 eV band gap (centered in the visible region) and optimally aligned conduction and valence bands for water reduction and oxidation, respectively. There is ongoing debate as to the roles of crystal structure, wurtzite (w-, hexagonal) vs zincblende (zb, cubic), and quantum confinement, in photocatalytic activity. Herein, we evaluated w- and zb-CdS quantum dots (QDs) of diameters of ∼3.2–3.3 nm for visible-light photocatalytic water reduction. wCdS QDs capped with trioctylphosphine oxide and tetradecylphosphonate were discovered to undergo an aggregation process upon sitting in the dark in hexane over 16 h, resulting in the observation of a second peak in the photoluminescence spectrum, red-shifted from the exciton peak, and correlating to the dramatically decreased photocatalytic water reduction activity: from 1221 (fresh) to 162 (16 h aged) μmol H2 h–1 g–1. In contrast, zbCdS QDs capped with oleate ligands did not exhibit a second emission peak and did not lose activity upon aging, producing 1458 μmol H2 h–1 g–1. The aggregation process is sufficiently modest that colloidal dispersibility is maintained. Nevertheless, aggregation leads to an enhancement of trap states and facilitates charge/energy transfer between QDs in the aggregates, limiting the availability of photogenerated electrons to the water reduction reaction. Thus, aggregation, even in small amounts, greatly affects the photophysical properties of wCdS QDs and limits their application in systems that demand photogenerated charge carriers’ availability and transport, such as photocatalysis, photovoltaics, and other light-harvesting devices.
中文翻译:

晶体结构对 CdS 量子点聚集的影响:对光物理性质和光催化析氢活性的影响
在当前对可再生能源的追求中,CdS 因其 2.4 eV 带隙(以可见光区域为中心)以及优化排列的导带和价带,成为太阳能驱动的光催化水分解制氢的有前途的成分,可减少水和水的消耗。氧化,分别。关于晶体结构、纤锌矿(w-,六方)与闪锌矿(zb,立方)以及量子限制在光催化活性中的作用一直存在争论。在此,我们评估了直径为~3.2-3.3 nm的w-和zb-CdS量子点(QD)用于可见光光催化减水的效果。发现用三辛基氧化膦和十四烷基膦酸酯封端的 wCdS QD 在黑暗中于己烷中放置 16 小时以上后会经历聚集过程,导致在光致发光光谱中观察到第二个峰,该峰从激子峰红移,并与光催化减水活性急剧下降:从1221(新鲜)到162(16小时陈化)μmol H 2 h –1 g –1 。相比之下,油酸配体封端的 zbCdS QD 没有表现出第二个发射峰,并且在老化时也没有失去活性,产生 1458 μmol H 2 h –1 g –1 .聚集过程足够温和,可以保持胶体分散性。然而,聚集导致陷阱态增强,并促进聚集体中量子点之间的电荷/能量转移,限制光生电子在水还原反应中的可用性。 因此,聚集,即使是少量,也会极大地影响 wCdS QD 的光物理性质,并限制其在需要光生电荷载流子可用性和传输的系统中的应用,例如光催化、光伏和其他光捕获设备。
更新日期:2024-06-26
中文翻译:

晶体结构对 CdS 量子点聚集的影响:对光物理性质和光催化析氢活性的影响
在当前对可再生能源的追求中,CdS 因其 2.4 eV 带隙(以可见光区域为中心)以及优化排列的导带和价带,成为太阳能驱动的光催化水分解制氢的有前途的成分,可减少水和水的消耗。氧化,分别。关于晶体结构、纤锌矿(w-,六方)与闪锌矿(zb,立方)以及量子限制在光催化活性中的作用一直存在争论。在此,我们评估了直径为~3.2-3.3 nm的w-和zb-CdS量子点(QD)用于可见光光催化减水的效果。发现用三辛基氧化膦和十四烷基膦酸酯封端的 wCdS QD 在黑暗中于己烷中放置 16 小时以上后会经历聚集过程,导致在光致发光光谱中观察到第二个峰,该峰从激子峰红移,并与光催化减水活性急剧下降:从1221(新鲜)到162(16小时陈化)μmol H 2 h –1 g –1 。相比之下,油酸配体封端的 zbCdS QD 没有表现出第二个发射峰,并且在老化时也没有失去活性,产生 1458 μmol H 2 h –1 g –1 .聚集过程足够温和,可以保持胶体分散性。然而,聚集导致陷阱态增强,并促进聚集体中量子点之间的电荷/能量转移,限制光生电子在水还原反应中的可用性。 因此,聚集,即使是少量,也会极大地影响 wCdS QD 的光物理性质,并限制其在需要光生电荷载流子可用性和传输的系统中的应用,例如光催化、光伏和其他光捕获设备。











































 京公网安备 11010802027423号
京公网安备 11010802027423号