当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigating the Origins of the pH-Dependent Oxidation of Glycerol over Platinum Using Differential Electrochemical Mass Spectrometry
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-06-25 , DOI: 10.1021/acs.jpcc.4c01372 Takuya Hamada 1, 2 , Abigail R. Circelli 2 , Hiroshi Inoue 1 , Clive A. Randall 3, 4 , Ezra L. Clark 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-06-25 , DOI: 10.1021/acs.jpcc.4c01372 Takuya Hamada 1, 2 , Abigail R. Circelli 2 , Hiroshi Inoue 1 , Clive A. Randall 3, 4 , Ezra L. Clark 2
Affiliation
|
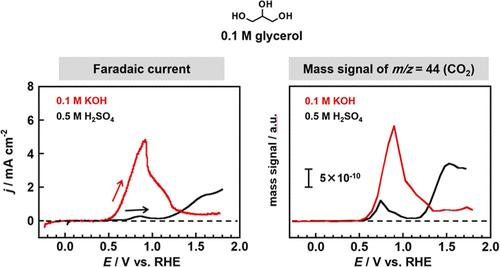
The glycerol oxidation reaction (GOR) to give CO2 could be harnessed to produce energy in a direct glycerol fuel cell. However, state-of-the-art electrocatalysts for this reaction exhibit poor voltage and current efficiency. Thus, there is a need to develop superior electrocatalysts for this process. However, rational electrocatalyst design is difficult due to the complicated reaction mechanism, which is poorly understood. In the present study, differential electrochemical mass spectrometry is utilized to investigate the mechanism of glycerol oxidation over Pt. This is accomplished by investigating the products formed during the electrochemical oxidation of glycerol and various potential reaction intermediates during cyclic voltammetry in acidic and alkaline media. Mass spectrometric cyclic voltammograms showed that the m/z = 44 signal, assigned to CO2, was detected during GOR over a Pt electrode in a 0.5 M H2SO4 solution. At electrode potentials greater than 1.0 V vs RHE, the m/z = 29 signal was also detected, suggesting the formation of formic acid (FA) by C–C bond cleavage reaction. The influence of electrolyte pH on the adsorption of glycerol and its various potential oxidation products on Pt(110) and Pt(100) active sites were investigated. Glycerol, glyceraldehyde, dihydroxyacetone, and FA were oxidized at an overpotential of ca. 0.7 V in 0.5 M H2SO4. However, the oxidation of most of the species potentially derived from glycerol required a higher overpotential (>1.0 V), providing an explanation for the low GOR kinetics in acidic media. Conversely, glyceric acid, hydroxypyruvic acid, mesoxalic acid, and glycolic acid were oxidized to CO2 at an overpotential of 0.5 V in alkaline media. Thus, alkaline media facilitates GOR kinetics by enabling intermediate oxidation products to remain adsorbed to the electrode surface.
中文翻译:

使用差示电化学质谱法研究铂上甘油的 pH 依赖性氧化的起源
产生 CO 2 的甘油氧化反应 (GOR) 可用于在直接甘油燃料电池中产生能量。然而,用于该反应的最先进的电催化剂表现出较差的电压和电流效率。因此,需要为该过程开发优异的电催化剂。然而,由于反应机理复杂,人们对其知之甚少,合理的电催化剂设计很困难。在本研究中,利用差示电化学质谱法研究 Pt 上甘油氧化的机理。这是通过研究甘油电化学氧化过程中形成的产物以及在酸性和碱性介质中循环伏安法期间形成的各种潜在反应中间体来实现的。质谱循环伏安图显示,在 GOR 过程中,在 0.5 M H 2 SO 4 SO 4 中为 0.7 V。然而,大多数可能源自甘油的物质的氧化需要更高的过电势(>1.0 V),这为酸性介质中的低 GOR 动力学提供了解释。相反,甘油酸、羟基丙酮酸、中草酸和乙醇酸在碱性介质中在0.5V的过电势下被氧化成CO 2 。 因此,碱性介质通过使中间氧化产物保持吸附在电极表面来促进 GOR 动力学。
更新日期:2024-06-27
中文翻译:

使用差示电化学质谱法研究铂上甘油的 pH 依赖性氧化的起源
产生 CO 2 的甘油氧化反应 (GOR) 可用于在直接甘油燃料电池中产生能量。然而,用于该反应的最先进的电催化剂表现出较差的电压和电流效率。因此,需要为该过程开发优异的电催化剂。然而,由于反应机理复杂,人们对其知之甚少,合理的电催化剂设计很困难。在本研究中,利用差示电化学质谱法研究 Pt 上甘油氧化的机理。这是通过研究甘油电化学氧化过程中形成的产物以及在酸性和碱性介质中循环伏安法期间形成的各种潜在反应中间体来实现的。质谱循环伏安图显示,在 GOR 过程中,在 0.5 M H 2 SO 4 SO 4 中为 0.7 V。然而,大多数可能源自甘油的物质的氧化需要更高的过电势(>1.0 V),这为酸性介质中的低 GOR 动力学提供了解释。相反,甘油酸、羟基丙酮酸、中草酸和乙醇酸在碱性介质中在0.5V的过电势下被氧化成CO 2 。 因此,碱性介质通过使中间氧化产物保持吸附在电极表面来促进 GOR 动力学。






































 京公网安备 11010802027423号
京公网安备 11010802027423号