当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cascade Synthesis of Pyrrolo[1,2-a]quinolines and Pyrrolo[2,1-a]isoquinolines via Formal [3 + 2]-Cycloaddition of Push–Pull Nitro Heterocycles with Carbonyl-Stabilized Quinolinium/Isoquinolinium Ylides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-25 , DOI: 10.1021/acs.joc.4c00455 Dmitry V Osipov 1 , Maxim R Demidov 1 , Alina A Artemenko 1 , Daria A Rashchepkina 1 , Pavel E Krasnikov 1 , Vitaly A Osyanin 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-25 , DOI: 10.1021/acs.joc.4c00455 Dmitry V Osipov 1 , Maxim R Demidov 1 , Alina A Artemenko 1 , Daria A Rashchepkina 1 , Pavel E Krasnikov 1 , Vitaly A Osyanin 1
Affiliation
|
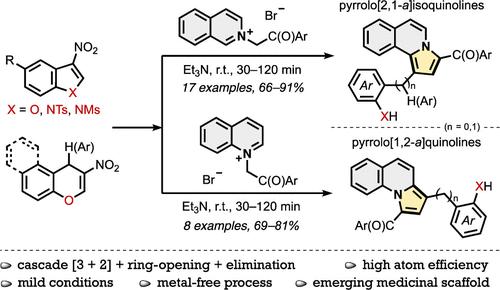
Various substituted pyrrolo[1,2-a]quinolines and pyrrolo[2,1-a]isoquinolines were synthesized in good to high yields by the Et3N-mediated reaction of push–pull 3-nitrobenzofurans or 1-Ts-/1-Ms-3-nitroindoles and precursors of carbonyl-stabilized quinolinium and isoquinolinium ylides as 1,3-dipole equivalents. These transformations proceed in a one-pot manner starting with the formal [3 + 2]-cycloaddition stage, which is accompanied by double dearomatization of both quinoline/isoquinoline and benzofuran/indole moieties, followed by ring-opening of cyclic intermediate formed and nitrous acid elimination sequence. [3 + 2]-Cycloadducts were isolated as the final products in cases of impossibility or difficulty of their enolization. The present protocol was successfully extended to 3-nitro-4H-chromene derivatives as push–pull dipolarophile component. Finally, using the method of competing reactions, the reactivity of the starting compounds was compared with each other.
中文翻译:

通过推拉硝基杂环与羰基稳定的喹啉鎓/异喹啉鎓叶立德的正式 [3 + 2]-环加成反应级联合成吡咯并[1,2-a]喹啉和吡咯并[2,1-a]异喹啉
通过 Et 3 N 介导的 3-硝基苯并呋喃或 1-Ts-/1 的推拉反应,以良好到高产率合成了各种取代的吡咯并[1,2- a ]喹啉和吡咯并[2,1 -a ]异喹啉-Ms-3-硝基吲哚以及羰基稳定的喹啉鎓和异喹啉鎓叶立德的前体(1,3-偶极当量)。这些转化以一锅方式进行,从正式的[3 + 2]-环加成阶段开始,伴随着喹啉/异喹啉和苯并呋喃/吲哚部分的双重脱芳构化,然后是形成的环状中间体和亚硝基的开环。酸消除顺序。在烯醇化不可能或困难的情况下,[3+2]-环氧化物被分离为最终产物。本协议已成功扩展到 3-硝基-4 H-色烯衍生物作为推拉亲偶极试剂组分。最后,采用竞争反应的方法,比较了起始化合物的反应活性。
更新日期:2024-06-25
中文翻译:

通过推拉硝基杂环与羰基稳定的喹啉鎓/异喹啉鎓叶立德的正式 [3 + 2]-环加成反应级联合成吡咯并[1,2-a]喹啉和吡咯并[2,1-a]异喹啉
通过 Et 3 N 介导的 3-硝基苯并呋喃或 1-Ts-/1 的推拉反应,以良好到高产率合成了各种取代的吡咯并[1,2- a ]喹啉和吡咯并[2,1 -a ]异喹啉-Ms-3-硝基吲哚以及羰基稳定的喹啉鎓和异喹啉鎓叶立德的前体(1,3-偶极当量)。这些转化以一锅方式进行,从正式的[3 + 2]-环加成阶段开始,伴随着喹啉/异喹啉和苯并呋喃/吲哚部分的双重脱芳构化,然后是形成的环状中间体和亚硝基的开环。酸消除顺序。在烯醇化不可能或困难的情况下,[3+2]-环氧化物被分离为最终产物。本协议已成功扩展到 3-硝基-4 H-色烯衍生物作为推拉亲偶极试剂组分。最后,采用竞争反应的方法,比较了起始化合物的反应活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号