当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective Synthesis of the HIV Protease Inhibitor Darunavir and Related Derivatives via a Titanium Tetrachloride-Mediated Asymmetric Glycolate Aldol Addition Reaction
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-25 , DOI: 10.1021/acs.joc.4c01057 Jordan M Witte 1 , Emmanuel Ayim 1 , Christopher J Sams 1 , Jasmine B Service 1 , Caitlyn C Kant 1 , Lillian Bambalas 1 , Daniel Wright 1 , Austin Carter 1 , Kelly Moran 1 , Isabella G Rohrig 1 , Gregory M Ferrence 1 , Shawn R Hitchcock 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-25 , DOI: 10.1021/acs.joc.4c01057 Jordan M Witte 1 , Emmanuel Ayim 1 , Christopher J Sams 1 , Jasmine B Service 1 , Caitlyn C Kant 1 , Lillian Bambalas 1 , Daniel Wright 1 , Austin Carter 1 , Kelly Moran 1 , Isabella G Rohrig 1 , Gregory M Ferrence 1 , Shawn R Hitchcock 1
Affiliation
|
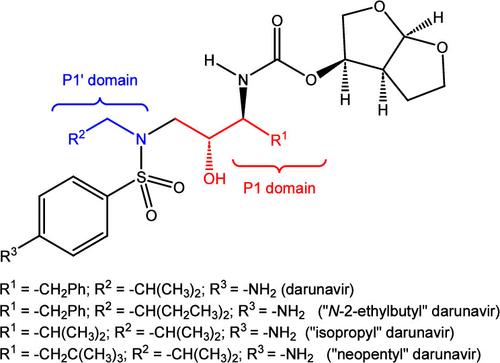
Darunavir is a potent HIV protease inhibitor that has been established as an effective tool in the fight against the progression of HIV/AIDS in the global community. The successful application of this drug has spurred the development of derivatives wherein strategic regions (e.g., P1, P1’, P2, and P2’) of the darunavir framework have been structurally modified. An alternate route for the synthesis of darunavir and three related P1 and P1’ derivatives has been developed. This synthetic pathway involves the use of a Crimmins titanium tetrachloride-mediated oxazolidine-2-thione-guided asymmetric glycolate aldol addition reaction. The resultant aldol adduct introduces the P1 fragment of darunavir via an aldehyde. Transamidation with a selected amine (isobutylamine or 2-ethyl-1-butylamine) to cleave the auxiliary yields an amide wherein the P1’ component is introduced. From this stage, the amide is reduced to the corresponding β-amino alcohol and the substrate is then bis-nosylated to introduce the requisite p-nitrobenzenesulfonamide component and activate the secondary alcohol for nucleophilic substitution. Treatment with sodium azide yielded the desired azides, and the deprotection of the p-methoxyphenoxy group is achieved with the use of ceric ammonium nitrate. Finally, hydrogenation to reduce both the aniline and azide functionalities with concurrent acylation yields darunavir and its derivatives.
中文翻译:

通过四氯化钛介导的不对称乙醇酸羟醛加成反应非对映选择性合成 HIV 蛋白酶抑制剂 darunavir 和相关衍生物
Darunavir 是一种有效的 HIV 蛋白酶抑制剂,已被确定为全球社区对抗 HIV/AIDS 进展的有效工具。该药物的成功应用刺激了衍生物的发展,其中 darunavir 框架的战略区域(例如 P1、P1'、P2 和 P2')已被结构修饰。已经开发了一种合成 darunavir 和三种相关 P1 和 P1' 衍生物的替代路线。该合成途径涉及使用 Crimmins 四氯化钛介导的噁唑烷-2-硫酮引导的不对称乙醇酸羟醛加成反应。所得的羟醛加合物通过醛引入 darunavir 的 P1 片段。用选定的胺(异丁胺或 2-乙基-1-丁胺)进行转酰胺反应以裂解助剂,得到酰胺,其中引入了 P1' 组分。从这个阶段开始,酰胺被还原为相应的 β-氨基醇,然后底物被双 no-nosylation 以引入必要的对硝基苯磺酰胺组分并激活仲醇以进行亲核取代。用叠氮化钠处理产生所需的叠氮化物,并且使用硝酸铈铵实现对甲氧基苯氧基的脱保护。最后,氢化以还原苯胺和叠氮化物官能团并同时酰化得到 darunavir 及其衍生物。
更新日期:2024-06-25
中文翻译:

通过四氯化钛介导的不对称乙醇酸羟醛加成反应非对映选择性合成 HIV 蛋白酶抑制剂 darunavir 和相关衍生物
Darunavir 是一种有效的 HIV 蛋白酶抑制剂,已被确定为全球社区对抗 HIV/AIDS 进展的有效工具。该药物的成功应用刺激了衍生物的发展,其中 darunavir 框架的战略区域(例如 P1、P1'、P2 和 P2')已被结构修饰。已经开发了一种合成 darunavir 和三种相关 P1 和 P1' 衍生物的替代路线。该合成途径涉及使用 Crimmins 四氯化钛介导的噁唑烷-2-硫酮引导的不对称乙醇酸羟醛加成反应。所得的羟醛加合物通过醛引入 darunavir 的 P1 片段。用选定的胺(异丁胺或 2-乙基-1-丁胺)进行转酰胺反应以裂解助剂,得到酰胺,其中引入了 P1' 组分。从这个阶段开始,酰胺被还原为相应的 β-氨基醇,然后底物被双 no-nosylation 以引入必要的对硝基苯磺酰胺组分并激活仲醇以进行亲核取代。用叠氮化钠处理产生所需的叠氮化物,并且使用硝酸铈铵实现对甲氧基苯氧基的脱保护。最后,氢化以还原苯胺和叠氮化物官能团并同时酰化得到 darunavir 及其衍生物。































 京公网安备 11010802027423号
京公网安备 11010802027423号