Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Synthesis of Urea: An In‐depth Investigation from Material Modification to Mechanism Analysis
Small ( IF 13.0 ) Pub Date : 2024-06-27 , DOI: 10.1002/smll.202403412 Jianghui Cao 1 , Fang Zhao 1 , Chengjie Li 2 , Qidong Zhao 1 , Liguo Gao 1 , Tingli Ma 3 , Hao Xu 4 , Xuefeng Ren 1 , Anmin Liu 1
Small ( IF 13.0 ) Pub Date : 2024-06-27 , DOI: 10.1002/smll.202403412 Jianghui Cao 1 , Fang Zhao 1 , Chengjie Li 2 , Qidong Zhao 1 , Liguo Gao 1 , Tingli Ma 3 , Hao Xu 4 , Xuefeng Ren 1 , Anmin Liu 1
Affiliation
|
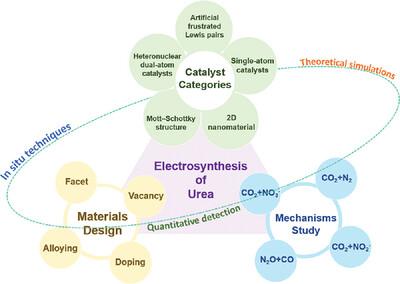
Industrial urea synthesis production uses NH3 from the Haber‐Bosch method, followed by the reaction of NH3 with CO2 , which is an energy‐consuming technique. More thorough evaluations of the electrocatalytic C−N coupling reaction are needed for the urea synthesis development process, catalyst design, and the underlying reaction mechanisms. However, challenges of adsorption and activation of reactant and suppression of side reactions still hinder its development, making the systematic review necessary. This review meticulously outlines the progress in electrochemical urea synthesis by utilizing different nitrogen (NO3 − , N2 , NO2 − , and N2 O) and carbon (CO2 and CO) sources. Additionally, it delves into advanced methods in materials design, such as doping, facet engineering, alloying, and vacancy introduction. Furthermore, the existing classes of urea synthesis catalysts are clearly defined, which include 2D nanomaterials, materials with Mott–Schottky structure, materials with artificially frustrated Lewis pairs, single−atom catalysts (SACs), and heteronuclear dual−atom catalysts (HDACs). A comprehensive analysis of the benefits, drawbacks, and latest developments in modern urea detection techniques is discussed. It is aspired that this review will serve as a valuable reference for subsequent designs of highly efficient electrocatalysts and the development of strategies to enhance the performance of electrochemical urea synthesis.
中文翻译:

尿素电催化合成:从材料改性到机理分析的深入研究
工业尿素合成生产使用哈伯-博世法中的NH3,然后NH3与CO2反应,这是一种耗能技术。尿素合成开发过程、催化剂设计和潜在反应机制需要对电催化 CN 偶联反应进行更彻底的评估。然而,反应物的吸附和活化以及副反应的抑制等挑战仍然阻碍其发展,因此有必要进行系统评价。这篇综述详细概述了利用不同氮源(NO3−、N2、NO2− 和 N2O)和碳源(CO2 和 CO)进行电化学尿素合成的进展。此外,它还深入研究了材料设计的先进方法,例如掺杂、晶面工程、合金化和空位引入。此外,现有的尿素合成催化剂类别也有明确的定义,包括二维纳米材料、莫特-肖特基结构材料、人工挫败路易斯对材料、单原子催化剂(SAC)和异核双原子催化剂(HDAC)。讨论了现代尿素检测技术的优点、缺点和最新发展的全面分析。希望这篇综述能为后续高效电催化剂的设计以及提高电化学尿素合成性能的策略的开发提供有价值的参考。
更新日期:2024-06-27
中文翻译:

尿素电催化合成:从材料改性到机理分析的深入研究
工业尿素合成生产使用哈伯-博世法中的NH3,然后NH3与CO2反应,这是一种耗能技术。尿素合成开发过程、催化剂设计和潜在反应机制需要对电催化 CN 偶联反应进行更彻底的评估。然而,反应物的吸附和活化以及副反应的抑制等挑战仍然阻碍其发展,因此有必要进行系统评价。这篇综述详细概述了利用不同氮源(NO3−、N2、NO2− 和 N2O)和碳源(CO2 和 CO)进行电化学尿素合成的进展。此外,它还深入研究了材料设计的先进方法,例如掺杂、晶面工程、合金化和空位引入。此外,现有的尿素合成催化剂类别也有明确的定义,包括二维纳米材料、莫特-肖特基结构材料、人工挫败路易斯对材料、单原子催化剂(SAC)和异核双原子催化剂(HDAC)。讨论了现代尿素检测技术的优点、缺点和最新发展的全面分析。希望这篇综述能为后续高效电催化剂的设计以及提高电化学尿素合成性能的策略的开发提供有价值的参考。











































 京公网安备 11010802027423号
京公网安备 11010802027423号