Cell ( IF 45.5 ) Pub Date : 2024-06-27 , DOI: 10.1016/j.cell.2024.05.058 Nicole Scott-Hewitt , Matthew Mahoney , Youtong Huang , Nils Korte , T. Yvanka de Soysa , Daniel K. Wilton , Emily Knorr , Kevin Mastro , Allison Chang , Allison Zhang , David Melville , Monica Schenone , Christina Hartigan , Beth Stevens
|
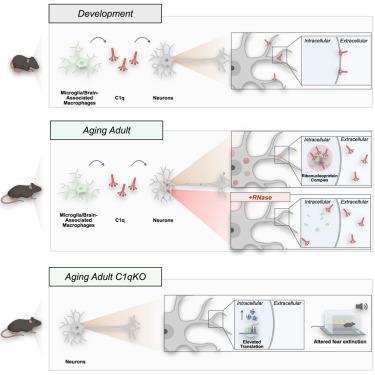
Neuroimmune interactions mediate intercellular communication and underlie critical brain functions. Microglia, CNS-resident macrophages, modulate the brain through direct physical interactions and the secretion of molecules. One such secreted factor, the complement protein C1q, contributes to complement-mediated synapse elimination in both developmental and disease models, yet brain C1q protein levels increase significantly throughout aging. Here, we report that C1q interacts with neuronal ribonucleoprotein (RNP) complexes in an age-dependent manner. Purified C1q protein undergoes RNA-dependent liquid-liquid phase separation (LLPS) in vitro, and the interaction of C1q with neuronal RNP complexes in vivo is dependent on RNA and endocytosis. Mice lacking C1q have age-specific alterations in neuronal protein synthesis in vivo and impaired fear memory extinction. Together, our findings reveal a biophysical property of C1q that underlies RNA- and age-dependent neuronal interactions and demonstrate a role of C1q in critical intracellular neuronal processes.
中文翻译:

小胶质细胞衍生的 C1q 整合到神经元核糖核蛋白复合物中并影响衰老大脑中的蛋白质稳态
神经免疫相互作用介导细胞间通讯,是关键大脑功能的基础。小胶质细胞是中枢神经系统驻留的巨噬细胞,通过直接的物理相互作用和分子分泌来调节大脑。补体蛋白 C1q 是此类分泌因子之一,在发育和疾病模型中有助于补体介导的突触消除,但大脑 C1q 蛋白水平在整个衰老过程中显着增加。在这里,我们报告 C1q 以年龄依赖性方式与神经元核糖核蛋白 (RNP) 复合物相互作用。纯化的 C1q 蛋白在体外经历 RNA 依赖性液-液相分离 (LLPS),而体内 C1q 与神经元 RNP 复合物的相互作用依赖于 RNA 和内吞作用。缺乏 C1q 的小鼠体内神经元蛋白质合成会发生年龄特异性改变,并且恐惧记忆消退受损。总之,我们的研究结果揭示了 C1q 的生物物理特性,它是 RNA 和年龄依赖性神经元相互作用的基础,并证明了 C1q 在关键的细胞内神经元过程中的作用。






































 京公网安备 11010802027423号
京公网安备 11010802027423号