Cell ( IF 45.5 ) Pub Date : 2024-06-27 , DOI: 10.1016/j.cell.2024.06.009 Lucas V. Cairo , Xiaoyu Hong , Martin B.D. Müller , Patricia Yuste-Checa , Chandhuru Jagadeesan , Andreas Bracher , Sae-Hun Park , Manajit Hayer-Hartl , F. Ulrich Hartl
|
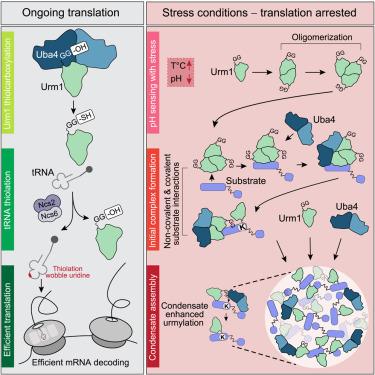
The ability of proteins and RNA to coalesce into phase-separated assemblies, such as the nucleolus and stress granules, is a basic principle in organizing membraneless cellular compartments. While the constituents of biomolecular condensates are generally well documented, the mechanisms underlying their formation under stress are only partially understood. Here, we show in yeast that covalent modification with the ubiquitin-like modifier Urm1 promotes the phase separation of a wide range of proteins. We find that the drop in cellular pH induced by stress triggers Urm1 self-association and its interaction with both target proteins and the Urm1-conjugating enzyme Uba4. Urmylation of stress-sensitive proteins promotes their deposition into stress granules and nuclear condensates. Yeast cells lacking Urm1 exhibit condensate defects that manifest in reduced stress resilience. We propose that Urm1 acts as a reversible molecular “adhesive” to drive protective phase separation of functionally critical proteins under cellular stress.
中文翻译:

泛素相关修饰剂 Urm1 调节压力依赖性凝聚态形成
蛋白质和 RNA 结合成相分离组件(例如核仁和应激颗粒)的能力是组织无膜细胞区室的基本原理。虽然生物分子凝聚物的成分通常都有详细记录,但其在压力下形成的机制仅被部分了解。在这里,我们在酵母中展示了泛素样修饰剂 Urm1 的共价修饰促进了多种蛋白质的相分离。我们发现,应激引起的细胞 pH 值下降会触发 Urm1 自我关联及其与靶蛋白和 Urm1 结合酶 Uba4 的相互作用。应激敏感蛋白的乌酰化促进它们沉积成应激颗粒和核凝聚物。缺乏 Urm1 的酵母细胞表现出凝聚缺陷,表现为应激恢复能力降低。我们提出 Urm1 作为一种可逆分子“粘合剂”,在细胞应激下驱动功能关键蛋白的保护性相分离。






































 京公网安备 11010802027423号
京公网安备 11010802027423号